Temporal dichotomy of neutrophil function in acute liver injury and repair
- PMID: 40606123
- PMCID: PMC12213964
- DOI: 10.1016/j.jhepr.2025.101417
Temporal dichotomy of neutrophil function in acute liver injury and repair
Abstract
Background & aims: Acetaminophen (APAP)-induced acute liver injury (APAP-ALI) is the leading cause of acute liver failure-induced death, with host innate immune responses driving outcomes. Neutrophils are activated and increased in APAP-ALI and reported to contribute to liver damage. However, neutrophil dysfunction in patients with acute liver failure is associated with non-survival, and recent reports highlight their importance in hepatic repair. Neutrophil-targeted therapies for APAP-ALI are hampered by this controversy and a lack of time-dependent investigation.
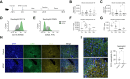
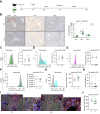
Methods: Hepatic neutrophils were depleted at different times in a wild-type mouse model of APAP-ALI. Fpr1 -/- mice, with reduced neutrophil activation, were also used. The impact of neutrophil depletion was interrogated during hepatic injury and repair after APAP-ALI, using serum biochemistry, liver and blood flow cytometry, liver histopathology, immunohistochemistry, ELISA, and NanoString analysis.
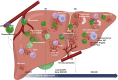
Results: Neutrophils contributed both to hepatic damage and repair after APAP-ALI. Early liver necrosis was reduced by neutrophil depletion (34% to 23%, p = 0.0018, n ≥10) and by reducing neutrophil functions (39% to 29%, p = 0.0279, n ≥11). By contrast, late neutrophil depletion resulted in markedly reduced liver repair (persistent necrosis 17% to 30%, p = 0.016, and higher serum alanine aminotransferase [1,221 to 3,725 IU/l, p = 0.0007, n ≥10]) and hepatocyte proliferation (decreased minichromosomal maintenance 2+ hepatocytes, 3% to 1%, p = 0.025, n = 10). Late neutrophil depletion reduced proliferation, growth factors, and angiogenesis transcripts (Mik6 fold change [FC] -6.322, p = 0.002; Socs2 FC -2.91, p = 0.01; vascular endothelial growth factor A FC -1.48, p = 0.01; n = 3). Similar transcript changes were identified when preventing formylated peptide receptor 1-mediated neutrophil activation, along with reduced extracellular matrix remodeling (Col12a1, FC -1.99, p = 0.0001; n ≥5). Finally, depleting neutrophils resulted in a hepatic proinflammatory monocyte/macrophage phenotype during repair stages, with increased proinflammatory-related transcripts and reduced reparative transcripts.
Conclusion: Recruited neutrophils contribute not only to hepatic damage early in APAP-ALI, but also to hepatic repair through a variety of pathways, including extracellular matrix remodeling, angiogenesis, hepatocyte proliferation, and promotion of an anti-inflammatory monocyte/macrophage phenotype.
Impact and implications: Novel therapies are required for APAP-ALI to improve patient outcomes. Neutrophil products and functions are potential targets for future therapies, but current literature controversy and a lack of time-dependent studies hinder progression. This study resolves the literature controversy, showing that neutrophils have time-dependent dichotomous roles in APAP-ALI. These insights highlight that early neutrophil-targeted interventions to reduce liver damage could be detrimental to subsequent patient recovery. Therefore, future research should aim to either elucidate isolated damaging functions or harness reparative functions of neutrophils for late-stage novel therapies for APAP-ALI.
Keywords: Acetaminophen; Extracellular matrix remodeling; Formylated-peptide-receptor 1; Hepatic; Inflammation; Macrophage; Monocyte; Paracetamol.
© 2025 The Authors.
Conflict of interest statement
There was no competing interests at the time of the experiments; however, LC, PSL, and SJF are shareholders of Resolution Therapeutics Ltd. a macrophage cell therapy developer. SJF is a scientific adviser for, and LC is an employee of, Resolution Therapeutics. AMK is a consultant for Resolution Therapeutics. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures





References
-
- Ostapowicz G., Fontana R.J., Schiodt F.V., et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. - PubMed
-
- Agrawal T., Maiwall R., Rajan V., et al. Higher circulating natural killer cells and lower lactate levels at admission predict spontaneous survival in non-acetaminophen induced acute liver failure. Clin Immunol. 2021;231 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

