Heterogeneous organocatalysis: the proline case
- PMID: 40606191
- PMCID: PMC12216369
- DOI: 10.1039/d5ra02331a
Heterogeneous organocatalysis: the proline case
Erratum in
-
Correction: Heterogeneous organocatalysis: the proline case.RSC Adv. 2025 Aug 21;15(36):29799. doi: 10.1039/d5ra90097b. eCollection 2025 Aug 18. RSC Adv. 2025. PMID: 40860071 Free PMC article.
Abstract
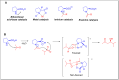
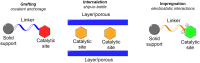
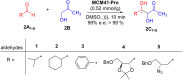
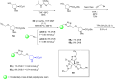
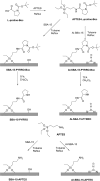
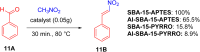
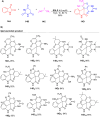
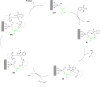
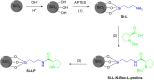
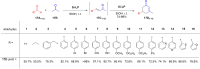
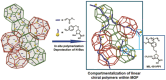
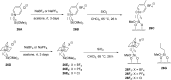
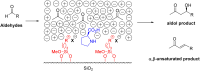
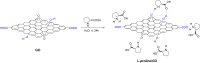
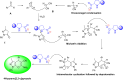
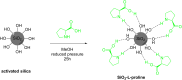
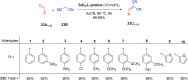
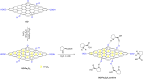
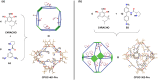
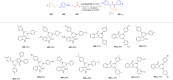
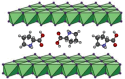
Asymmetric catalysis has allowed organic chemists to synthesize chiral molecules, such as amino acids, nucleic acids, sugars, and drugs. To achieve this, it is common to use a chiral catalyst to selectively accelerate the reaction. In the early days, it was believed that there were two main types of effective asymmetric catalysts: enzyme complexes and transition metal complexes. However, with the emergence of organocatalysis and the consequent introduction of a new category of highly effective asymmetric catalysts based solely on organic compounds, there was a revolution in the field of asymmetric catalysis. An excellent example of this innovation is l-proline, a fascinating molecule that demonstrates the transformative impact of organocatalysis. Organocatalysis presents itself as a simpler synthetic tool than other methodologies, however, despite being widely used in the research environment, it has not yet reached large-scale production. This gap occurs mainly due to the methodology working in a homogeneous phase together with the rest of the reagents involved. Although this does not present a problem on a laboratory scale, the recovery and reuse of the catalyst can be an obstacle in industrial processes. In recent years, with a view to some industrial uses, there has been an effort to make the immobilization (also called heterogenization) of organocatalysts possible. Such modified systems have broad catalytic applications in several organic transformations. Taking this into consideration, this review has the general objective of investigating the application of different supports in the immobilization of the classical organocatalyst l-proline, synthesized and characterized by three different methodologies, namely: impregnation, intercalation and grafting.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


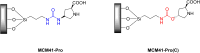
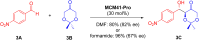



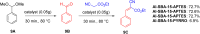


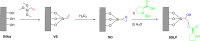
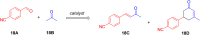




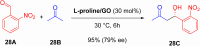
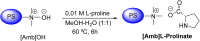

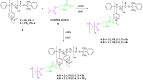
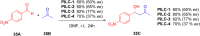
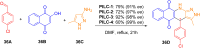
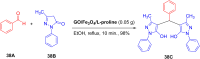
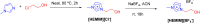
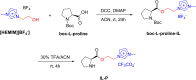
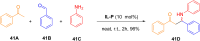


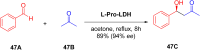


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

