Exploring bone-tumor interactions through 3D in vitro models: Implications for primary and metastatic cancers
- PMID: 40606222
- PMCID: PMC12219387
- DOI: 10.1016/j.jbo.2025.100698
Exploring bone-tumor interactions through 3D in vitro models: Implications for primary and metastatic cancers
Abstract
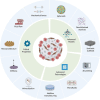
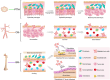
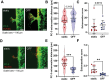
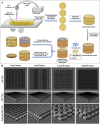
Bone tissue serves as a perfect hosting site where metastatic cancer cells of the most prevalent cancer types, such as prostate and breast cancers, as well as the native bone sarcomas, can further proliferate, advancing the disease stage with the consequential decline of the patient's prognosis. Understanding how the bone niche interacts with tumor cells and the mechanisms leading to drug resistance is a crucial step for enabling the identification of effective cancer therapies. Nevertheless, bone tumor research and the development of new effective anticancer drugs have been hampered for a long time due to the limitations of preclinical models. Traditional 2D cultures and animal models have failed to accurately replicate the human bone cancer microenvironment, driving researchers to develop 3D in vitro bone models using tissue-engineered bone constructs and advanced technologies like microfluidics and additive manufacturing. While a complete reproduction of the bone tumor microenvironment (TME), including all relevant cell types, stromal elements, and biophysical cues, remains elusive, targeted inclusion of key components has advanced the physiological relevance of these models. The following review evaluates the biomimetic approaches that have been used to recapitulate the bone TME through 3D in vitro models, with particular attention to recent studies aimed at more accurately mimicking the complexity of bone TME, highlighting future directions and the advancements required to overcome present limitations.
Keywords: 3D models; Bone tumors; In vitro; Metastatic cancers; Tumor microenvironment.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Surveillance of Barrett's oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.Health Technol Assess. 2006 Mar;10(8):1-142, iii-iv. doi: 10.3310/hta10080. Health Technol Assess. 2006. PMID: 16545207
-
Engineered 3D ex vivo models to recapitulate the complex stromal and immune interactions within the tumor microenvironment.Biomaterials. 2024 Mar;305:122428. doi: 10.1016/j.biomaterials.2023.122428. Epub 2023 Dec 19. Biomaterials. 2024. PMID: 38147743 Free PMC article. Review.
References
-
- Chaffer C.L., Weinberg R.A. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. - PubMed
-
- Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006;12(20):6243s–s6249. - PubMed
-
- Sathiakumar N., Delzell E., Morrisey M.A., Falkson C., Yong M., Chia V., Blackburn J., Arora T., Kilgore M. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis. 2011;14(2):177–183. - PubMed
-
- J. Ferlay, I. Soerjomataram, M. Ervik, R. Dikshit, S. Eser, C. Mathers, M. Rebelo, D. Parkin, D. Forman, F. Bray, GLOBOCAN 2012 v1. 0, Cancer incidence and mortality worldwide: IARC CancerBase 11 (2013). - PubMed
Publication types
LinkOut - more resources
Full Text Sources

