Unravelling γD-crystallin aggregation pathway to understand cataract formation using fluorescence correlation spectroscopy
- PMID: 40606473
- PMCID: PMC12221310
Unravelling γD-crystallin aggregation pathway to understand cataract formation using fluorescence correlation spectroscopy
Abstract
Purpose: To characterize the aggregation behavior of the γD-crystallin protein in an acidic environment with a focus on the formation of intermediate species. The research employs fluorescence correlation spectroscopy to unravel the intricate molecular events leading to aggregation, contributing to a comprehensive understanding of cataract formation.
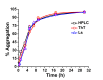
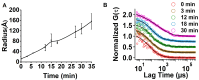
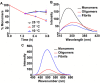
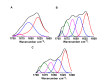
Methods: The kinetics of γD-crystallin protein aggregation were studied with a reversed-phase high-performance liquid chromatography sedimentation assay, a ThT binding assay, and light scattering. We used fluorescence correlation spectroscopy (FCS) to recognize intermediate aggregate species and characterized them with Fourier transform infrared spectroscopy (FTIR). Further, the morphologic characterization of aggregates was done by transmission electron microscopy (TEM), and their hydrophobic characteristics were analyzed using the 8-anilino-1-naphthalenesulfonic acid binding assay.
Results: A negligible lag phase was observed in the aggregation kinetic experiments of the γD-crystallin protein. Pentamer, 25-mer, and higher oligomer intermediates were formed on the aggregation pathway. Conformation studies by FCS and FTIR have shown that oligomers are rich in cross-β sheet and random coil structure; however, they constitute more α-helix and less cross-β sheet structure than fibrils. TEM analysis revealed the approximate size of oligomers (diameter ~10 nm), protofibrils (~15 nm), and fibrils (~15 to ~35 nm).
Conclusions: In this study, we reported the presence of various intermediate aggregate species formed on the aggregation pathway of γD-crystallin protein at low pH. This will open new areas of research in understanding the detailed aggregation mechanism and aggregation hotspot within unfolded γD-crystallin monomers. The insights gained will also pave the way for future research in the realm of amyloid formation in cataract.
Copyright © 2025 Molecular Vision.
Figures


References
-
- Lam D, Rao SK, Ratra V, Liu Y, Mitchell P, King J, Tassignon MJ, Jonas J, Pang CP, Chang DF. Cataract. Nat Rev Dis Primers. 2015;1:15014. - PubMed
-
- Morishita H, Mizushima N. Autophagy in the lens. Exp Eye Res. 2016;144:22–8. - PubMed
-
- Zhao L, Chen X-J, Zhu J, Xi Y-B, Yang X, Hu L-D, Ouyang H, Patel SH, Jin X, Lin D, Wu F, Flagg K, Cai H, Li G, Cao G, Lin Y, Chen D, Wen C, Chung C, Wang Y, Qiu A, Yeh E, Wang W, Hu X, Grob S, Abagyan R, Su Z, Tjondro HC, Zhao XJ, Luo H, Hou R, Jefferson J, Perry P, Gao W, Kozak I, Granet D, Li Y, Sun X, Wang J, Zhang L, Liu Y, Yan YB, Zhang K. Lanosterol reverses protein aggregation in cataracts. Nature. 2015;523:607–11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

