Stem cells in the treatment of myocardial injury-induced cardiomyopathy: mechanisms and efficient utilization strategies
- PMID: 40606613
- PMCID: PMC12213730
- DOI: 10.3389/fphar.2025.1600604
Stem cells in the treatment of myocardial injury-induced cardiomyopathy: mechanisms and efficient utilization strategies
Abstract
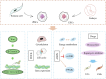
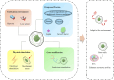
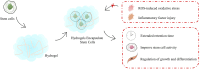
Cardiac tissue injury and repair have always been a research hotspot in the field of cardiovascular disease. Limited and lost myocardial cells are non-renewable, and the current clinical treatment effect is still poor. The stem cells-based treatment strategy for cardiomyopathy is expected to solve the current treatment pain points. A variety of stem cells have the potential to differentiate into cardiomyocytes and form cardiac tissue, and the strong paracrine activity of stem cells also plays an important role in the regulation of inflammation, oxidative stress and cardiomyocyte apoptosis in cardiac tissue. Limited by the survival rate and stem cells activity after stem cells transplantation, the effect of stem cells therapy on cardiomyopathy is still not ideal. Pretreatment of stem cells or genetic modification to enhance the adaptability of stem cells to the environment, or the use of new biomaterials to assist stem cells transplantation is an effective optimization scheme and significantly enhances the therapeutic effect of stem cells therapy for cardiomyopathy. In this review, the types of stem cells widely studied in the treatment of cardiomyopathy, the role of stem cells in the treatment of cardiomyopathy, and how to efficiently use stem cells to treat cardiomyopathy are described in detail, which provides a theoretical basis for promoting the preclinical research and clinical transformation of stem cell therapy for cardiomyopathy.
Keywords: cardiomyopathy; cell differentiation; hydrogels; paracrine effect; stem cells.
Copyright © 2025 Yang, Yue, He, Xiong, Luo and Hou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
A systematic overview of chemotherapy effects in acute myeloid leukaemia.Acta Oncol. 2001;40(2-3):231-52. doi: 10.1080/02841860151116321. Acta Oncol. 2001. PMID: 11441935
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
-
The health economics of insulin therapy: How do we address the rising demands, costs, inequalities and barriers to achieving optimal outcomes.Diabetes Obes Metab. 2025 Jul;27 Suppl 5(Suppl 5):24-35. doi: 10.1111/dom.16488. Epub 2025 Jun 4. Diabetes Obes Metab. 2025. PMID: 40464081 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Ahmad F. S., Jin Y., Grassam-Rowe A., Zhou Y., Yuan M., Fan X., et al. (2023). Generation of cardiomyocytes from human-induced pluripotent stem cells resembling atrial cells with ability to respond to adrenoceptor agonists. Philos. Trans. R. Soc. Lond B Biol. Sci. 378 (1879), 20220312. 10.1098/rstb.2022.0312 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

