Oral Angiotensin-(1-7) formulation after established elastase-induced emphysema suppresses inflammation and restores lung architecture
- PMID: 40606618
- PMCID: PMC12213791
- DOI: 10.3389/fphar.2025.1540475
Oral Angiotensin-(1-7) formulation after established elastase-induced emphysema suppresses inflammation and restores lung architecture
Abstract
Background: Chronic obstructive pulmonary disease (COPD), a prevalent age-related condition, ranks among the leading causes of global mortality. It is characterized by chronic inflammation, cellular senescence, and irreversible lung tissue damage, with no curative treatments currently available. Angiotensin-(1-7) [Ang-(1-7)] has demonstrated anti-inflammatory and regenerative potential in preclinical models. This study aimed to investigate the therapeutic effects of oral Ang-(1-7) on senescence, inflammation, and tissue regeneration in a model of elastase-induced pulmonary emphysema.
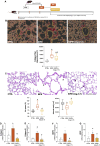
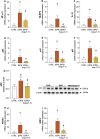
Methods: Male C57BL/6 mice were subjected to emphysema induction through three intratracheal instillations of porcine pancreatic elastase (PPE). One week after the final elastase instillation, the mice were treated with Ang-(1-7) encapsulated in hydroxypropyl-β-cyclodextrin to enhance its bioavailability. The treatment was administered daily for 4 weeks. Histological assessments, gene expression analysis, and protein quantification through Western blot were performed to evaluate lung architecture, inflammation, and senescence markers.
Results: The results showed that elastase exposure led to significant lung damage, including enlarged airspaces, increased collagen deposition and upregulated expression of collagen I/III and MMP9. Markers of inflammation and senescence were significantly elevated in the untreated emphysema group. However, treatment with Ang-(1-7) reversed these changes, reducing collagen deposition, restoring alveolar structure, and suppressing inflammation and senescence. Additionally, Ang-(1-7) modulated key signaling pathways, reactivating the Wnt/β-catenin pathway for tissue regeneration and inhibiting NF-κB activation, critical for inflammation suppression.
Conclusion: These findings suggest that Ang-(1-7), when administered after disease establishment, demonstrates potential to reverse structural lung damage and suppress chronic inflammation in experimental models, indicating a promising direction for future translational and clinical research in COPD.
Keywords: NF-κB modulation; Wnt/β-catenin pathway; alveolar regeneration; cellular senescence; chronic inflammation.
Copyright © 2025 Magalhaes, Villacampa, Rodrigues-Machado, Campagnole-Santos, Souza Santos, Sánchez-Ferrer and Peiró.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Psychological therapies for the treatment of anxiety disorders in chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2017 Mar 21;3(3):CD010673. doi: 10.1002/14651858.CD010673.pub2. Cochrane Database Syst Rev. 2017. PMID: 28322440 Free PMC article.
-
Efficacy and safety of ensifentrine in treatment of COPD: a systematic review and meta-analysis of clinical trials.Ther Adv Respir Dis. 2025 Jan-Dec;19:17534666251347775. doi: 10.1177/17534666251347775. Epub 2025 Jun 20. Ther Adv Respir Dis. 2025. PMID: 40538231 Free PMC article.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.Health Technol Assess. 2001;5(26):1-149. doi: 10.3310/hta5260. Health Technol Assess. 2001. PMID: 11701099
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2017 May 23;5(5):CD011425. doi: 10.1002/14651858.CD011425.pub2. Cochrane Database Syst Rev. 2017. PMID: 28535331 Free PMC article.
Cited by
-
The role of the classical renin-angiotensin system and angiotensin-converting enzyme 2/Ang(1-7)/Mas axis in pulmonary fibrosis.Front Med (Lausanne). 2025 Jul 29;12:1615991. doi: 10.3389/fmed.2025.1615991. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40800134 Free PMC article. Review.
References
-
- Bastos A. C., Magalhães G. S., Gregório J. F., Matos N. A., Motta-Santos D., Bezerra F. S., et al. (2020). Oral formulation angiotensin-(1-7) therapy attenuates pulmonary and systemic damage in mice with emphysema induced by elastase. Immunobiology. 225 (2), 151893. 10.1016/j.imbio.2019.12.002 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

