Application of non-invasive prenatal testing for fetal chromosomal disorders in low-risk pregnancies: a follow-up study in central China
- PMID: 40606667
- PMCID: PMC12213843
- DOI: 10.3389/fgene.2025.1574775
Application of non-invasive prenatal testing for fetal chromosomal disorders in low-risk pregnancies: a follow-up study in central China
Abstract
Objective: To evaluate the performance and screening value of noninvasive prenatal testing (NIPT) in low-risk pregnancies.
Methods: A retrospective analysis was conducted on 60193 low-risk pregnancies over the last 5 years. Whole-genome sequencing of maternal plasma cell-free DNA was performed using next-generation sequencing. NIPT-positive results were confirmed using amniocentesis with karyotyping and/or copy number variation sequencing and chromosomal microarray analysis. Fetal outcomes were assessed using electronic medical records or telephone calls.
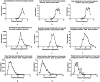
Results: Overall, 598 (0.99%) NIPT-positive cases were identified. The distribution of chromosomal abnormalities included sex chromosome aneuploidies (SCAs; 55.85%), rare autosomal aneuploidies (RAAs; 20.40%), copy number variations (CNVs; 11.20%), trisomy 21 (T21; 6.86%), trisomy 13 (T13; 4.01%), and trisomy 18 (T18; 1.67%). A total of 572 (95.65%) patients with NIPT-positive results underwent amniocentesis, and 55.77% (319/572) cases were confirmed. The positive predictive values (PPV) for T21, T18, T13, SCAs, RAAs, and CNVs were 87.50%, 60.00%, 34.78%, 58.97%, 32.50%, and 69.70%, respectively, and the PPV for the trisomy was higher than that for the X-monomer in SCAs. NIPT-positive results for RAAs were common in T8, T10, T16 and T20, but T16 was the most common true positive result, accounting for 33.33% (13/39) of the cases. The termination rates of true-positive pregnancies were 100% (T21, T18 and T13), 79.49% (RAAs), 67.39% (CNVs) and 78.07% (SCAs).
Conclusion: This study highlights the importance of genome-wide screening based on NIPT in low-risk pregnancies. Prenatal screening by NIPT has a high sensitivity and PPV. Moreover, it can greatly reduce invasive procedures and birth defects.
Keywords: aneuploidy; copy number variations (CNVs); follow-up; low-risk; non-invasive prenatal testing (NIPT).
Copyright © 2025 Huang, Xu, Chen, Fan and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Genomics-based non-invasive prenatal testing for detection of fetal chromosomal aneuploidy in pregnant women.Cochrane Database Syst Rev. 2017 Nov 10;11(11):CD011767. doi: 10.1002/14651858.CD011767.pub2. Cochrane Database Syst Rev. 2017. PMID: 29125628 Free PMC article.
-
[Prenatal diagnosis and analysis of fetuses with false-positive NIPT results caused by sex chromosomal abnormalities in pregnant women].Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2025 May 10;42(5):525-531. doi: 10.3760/cma.j.cn511374-20250127-00053. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2025. PMID: 40623922 Chinese.
-
A retrospective analysis of 6942 amniocentesis cases.BMC Pregnancy Childbirth. 2025 Aug 12;25(1):838. doi: 10.1186/s12884-025-07992-4. BMC Pregnancy Childbirth. 2025. PMID: 40797307 Free PMC article.
-
Non-invasive prenatal testing (NIPT) in twin pregnancies affected by early single fetal demise: A systematic review of NIPT and vanishing twins.Prenat Diagn. 2023 Jun;43(7):829-837. doi: 10.1002/pd.6388. Epub 2023 May 31. Prenat Diagn. 2023. PMID: 37226326
-
Analysis of cell-free fetal DNA in maternal blood for detection of trisomy 21, 18 and 13 in a general pregnant population and in a high risk population - a systematic review and meta-analysis.Acta Obstet Gynecol Scand. 2017 Jan;96(1):7-18. doi: 10.1111/aogs.13047. Epub 2016 Dec 9. Acta Obstet Gynecol Scand. 2017. PMID: 27779757
References
-
- Acreman M. L., Bussolaro S., Raymond Y. C., Fantasia I., Rolnik D. L., Da Silva Costa F. (2023). The predictive value of prenatal cell-free DNA testing for rare autosomal trisomies: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 228 (3), 292–305.e6. 10.1016/j.ajog.2022.08.034 - DOI - PubMed
-
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins — Obstetrics, Committee on Genetics, and Society for Maternal-Fetal MedicineCommittee on GeneticsSociety for Maternal-Fetal Medicine (2020). Screening for fetal chromosomal abnormalities: ACOG Practice bulletin, number 226. Obstet. Gynecol. 136 (4), e48–e69. 10.1097/AOG.0000000000004084 - DOI - PubMed
-
- Bakkeren I. M., Henneman L., van Vliet-Lachotzki E. H., Martin L., Gitsels-van der Wal J. T., Polak M. G., et al. (2024). Psychological impact of additional findings detected by genome-wide Non-Invasive Prenatal Testing (NIPT): TRIDENT-2 study. Eur. J. Hum. Genet. 32 (3), 302–308. 10.1038/s41431-023-01504-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

