Randomized controlled trial of prednisone in treating acute subjective tinnitus patients with normal pure-tone thresholds
- PMID: 40606757
- PMCID: PMC12221516
- DOI: 10.1016/j.isci.2025.112840
Randomized controlled trial of prednisone in treating acute subjective tinnitus patients with normal pure-tone thresholds
Abstract
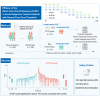
The effectiveness of steroid therapy for acute subjective tinnitus (AST) without sudden hearing loss remains controversial. This randomized controlled trial evaluated the effectiveness of short-term oral prednisone (STOP) in patients with AST. The STOP group received a 14-day tapering prednisone for the initial 14 days, and both groups took Ginkgo biloba extract throughout the 3-month follow-up period. The primary outcome was the 12-week change in Tinnitus Handicap Inventory (THI) scores, assessed via intention-to-treat analysis. At the 12-week follow-up, the STOP medication significant reduced THI scores (-27 · 34 [95% CI, -31 · 14 to -23 · 55]) versus control (-15 · 37 [95% CI, -18 · 5 to -12 · 23]), with a mean difference of -11 · 97 points (95% CI, -16 · 85 to -7·09; p < 0 · 0001). Sensitivity analyses and post hoc analyses corroborated these findings. In summary, the STOP treatment plus Ginkgo biloba could effectively lessen the self-reported tinnitus severity in patients with AST.
Keywords: Clinical neuroscience; Therapeutics.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
LinkOut - more resources
Full Text Sources

