In Silico Lung Deposition Profiles of Three Single-Inhaler Triple Therapies in Patients with COPD Using Functional Respiratory Imaging
- PMID: 40606813
- PMCID: PMC12214429
- DOI: 10.2147/COPD.S510214
In Silico Lung Deposition Profiles of Three Single-Inhaler Triple Therapies in Patients with COPD Using Functional Respiratory Imaging
Abstract
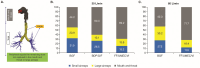
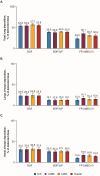
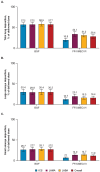
Introduction: Inhalation is the foundational route for administering pharmacological treatments for chronic obstructive pulmonary disease (COPD). Given the relevance of both large and small airways in COPD, lung distribution deposition characteristics of different inhaler options could influence treatment effects. This study evaluated the total deposition of 3 single-inhaler triple therapies in the large and small airways.
Methods: This study assessed lung deposition of different inhalers using in silico functional respiratory imaging from 20 patients with COPD. The first part evaluated budesonide/glycopyrronium/formoterol fumarate dihydrate (BGF) pressurized metered-dose inhaler (pMDI), beclomethasone dipropionate/glycopyrronium/formoterol fumarate dihydrate (BDP/G/F) pMDI, and fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) dry powder inhaler (DPI) at 30 L/min. The second part assessed BGF pMDI and FF/UMEC/VI DPI at 60 L/min.
Results: All 3 inhalers had different in silico total lung and large and small airways deposition profiles at 30 L/min, with BGF pMDI (54.8% to 57.7%) demonstrating a higher deposition profile across each therapeutic component versus BDP/G/F pMDI (38.6% to 40.5%) and FF/UMEC/VI DPI (24.0% to 36.1%), respectively. Similarly, at 60 L/min, the total deposition of all 3 components of BGF pMDI (57.2% to 58.5%) remained higher compared with FF/UMEC/VI DPI (19.8% to 34.1%).
Conclusion: These findings provide quantitative insights into relative lung deposition distribution profiles and efficiency of delivery for 3 inhaler options. Further research is needed to understand if these deposition patterns could translate into real-world clinical effectiveness differences.
Keywords: chronic obstructive pulmonary disease; functional respiratory imaging; single-inhaler triple therapy; small airways.
© 2025 Singh et al.
Conflict of interest statement
Dave Singh reports receipt of personal fees from Adovate, Aerogen, Almirall, Apogee, Arrowhead, AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Cipla, CONNECT Biopharm, Covis, CSL Behring, DevPro Biopharma LCC, Elpen, Empirico, EpiEndo, Genentech, Generate Biomedicines, GSK, Glenmark, Kamada, Kinaset Therapeutics, Kymera, Menarini, MicroA, OM Pharma, Orion, Pieris Pharmaceuticals, Pulmatrix, Revolo, Roivant Sciences, Sanofi, Synairgen, Tetherex, Teva, Theravance Biopharma, Upstream, and Verona Pharma. Nicolas Roche reports receipt of grants from Boehringer Ingelheim, GSK, Novartis, and Pfizer; personal consulting fees from AstraZeneca, Austral, Bayer, Biosency, Boehringer Ingelheim, Chiesi, GSK, Novartis, Pfizer, Sanofi, and Teva; personal payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Menarini, MSD, Novartis, Pfizer, Sanofi, Teva, and Zambon; and support for attending meetings and/or travel from AstraZeneca, Chiesi, and GSK. Libo Wu and Jonathan Marshall are employees of AstraZeneca and may hold stock and/or stock options in the company. Hosein Sadafi, Jan De Backer, and Navid Monshi Tousi are employees of FLUIDDA, which received funding from AstraZeneca for this study. The authors report no other conflicts of interest in this work.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

