Acellular porcine placental membranes as a novel biomaterial for tissue repair applications
- PMID: 40606914
- PMCID: PMC12213565
- DOI: 10.3389/fbioe.2025.1606615
Acellular porcine placental membranes as a novel biomaterial for tissue repair applications
Abstract
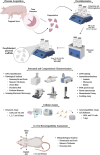
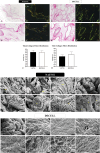
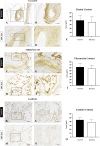
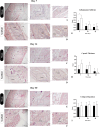
Biological dressings derived from the extracellular matrix (ECM) of human placental tissues have proven effective in treating complex skin wounds and other anatomical sites, offering potential for new therapeutic applications. However, the use of human tissues is limited by ethical and biosafety concerns, restricting large-scale production. To address this, biomaterials from placentas of livestock animals offer a cost-effective, accessible alternative without harming animal welfare. Given pigs' large-scale production, short gestation periods, and abundant material availability, this study aimed to produce, characterize, and validate acellular biomembranes derived from decellularized porcine allantochorion for tissue repair. Placental fragments from Duroc sows were decellularized using a protocol involving immersion and orbital shaking in 0.1% SDS and 0.5% Triton X-100, followed by low-frequency ultrasonication. Accelularity was confirmed by total genomic DNA quantification and H&E and DAPI staining for nuclear visualization. Membrane structure and composition were analyzed using histological, immunohistochemical methods, and scanning electron microscopy. Spectroscopic analyses detected physicochemical changes in placental ECM, and biomechanical testing assessed membrane strength and stiffness. Biological functionality was validated through in vitro cell viability and adhesion assays with canine endothelial progenitor cells and L929 murine fibroblasts. In vivo biocompatibility was tested by subcutaneously implanting the biomaterial in rats for histopathological evaluation. Results showed efficient decellularization, with preserved ECM structure. The scaffolds were cytocompatible, supporting cell adhesion and high viability. In vivo testing revealed no immune rejection, confirming biocompatibility and biodegradability. In conclusion, acellular porcine placental biomembranes have the necessary characteristics to be explored as scaffolds for tissue engineering and novel repair therapies.
Keywords: biocompatibility; biomaterial; decellularization; placenta; porcine.
Copyright © 2025 Almeida, Lima, Gibbin, Lopomo, Bergamo, da Silva, Santos, Silva, D’Onofrio, dos Santos, Silva, da Silva, Fuzeti, Candian, Nesiyama, Damin, Oliveira, Saavedra, de Almeida, de Almeida, Rinaldi, Sato, Baesso, Hernandes, Meirelles, Rici, Maria, Mathias and Carreira.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






Similar articles
-
Greener Decellularization of Porcine Auricular Cartilage Using Supercritical Technology and Different Pretreatments for Application in Tissue Engineering.ACS Biomater Sci Eng. 2025 Jul 14;11(7):4293-4304. doi: 10.1021/acsbiomaterials.4c02155. Epub 2025 Jun 19. ACS Biomater Sci Eng. 2025. PMID: 40534570 Free PMC article.
-
Assessing the effects of bladder decellularization protocols on extracellular matrix (ECM) structure, mechanics, and biology.J Pediatr Urol. 2024 Oct;20(5):843-850. doi: 10.1016/j.jpurol.2024.06.002. Epub 2024 Jun 8. J Pediatr Urol. 2024. PMID: 38945790 Free PMC article.
-
Bovine Placental Cotyledon-Derived Hydrogel: Methodology to Produce a Substrate for Tissue Engineering.Tissue Eng Part C Methods. 2025 Jul;31(7):271-279. doi: 10.1177/19373341251360957. Epub 2025 Jul 14. Tissue Eng Part C Methods. 2025. PMID: 40657703
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
References
-
- Alizadeh S., Nasiri M., Saraei M., Zahiri M., Khosrowpour Z., Sineh Sepehr K., et al. (2024). Optimization of an affordable and efficient skin allograft composite with excellent biomechanical and biological properties suitable for the regeneration of deep skin wounds: a preclinical study. ACS Appl. Bio Mater. 7 (11), 7378–7390. 10.1021/ACSABM.4C01016 - DOI - PubMed
-
- Almeida G. H. D. R., da Silva-Júnior L. N., Gibin M. S., dos Santos H., de Oliveira Horvath-Pereira B., Pinho L. B. M., et al. (2023). Perfusion and ultrasonication produce a decellularized porcine whole-ovary scaffold with a preserved microarchitecture. Cells 12 (14), 1864. 10.3390/CELLS12141864 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

