Effect of rapamycin nanoparticles in an animal model of primary biliary cholangitis
- PMID: 40606922
- PMCID: PMC12210173
- DOI: 10.4254/wjh.v17.i6.104073
Effect of rapamycin nanoparticles in an animal model of primary biliary cholangitis
Abstract
Background: Primary biliary cholangitis (PBC) is a chronic autoimmune-mediated cholestatic liver disease. Nanoparticles encapsulating rapamycin (ImmTOR) suppress adaptive immune responses and induce the hepatic tolerogenic immune response.
Aim: To investigate the effects of ImmTOR in PBC mouse models.
Methods: PBC models were induced in C57BL/6 mice by two immunizations of 2-octynoic acid-coupled bovine serum albumin at two-week intervals, and polycytidylic acid every three days. The PBC mouse models were separated into the treatment group and the control group. The levels of alkaline phosphatase (ALP) and alanine aminotransferase in the mice were detected using an automatic biochemical analyzer. Liver and spleen mononuclear cells were analyzed by flow cytometry, and serum anti-mitochondrial antibodies (AMA) and the related cytokines were analyzed by enzyme-linked immunosorbent assay. Liver histopathology was examined by hematoxylin and eosin staining and scored.
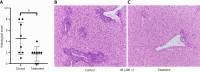
Results: After treatment with ImmTOR, the ALP level was significantly decreased (189.60 U/L ± 27.25 U/L vs 156.00 U/L ± 17.21 U/L, P < 0.05), the level of AMA was reduced (1.28 ng/mL ± 0.27 ng/mL vs 0.56 ng/mL ± 0.07 ng/mL, P < 0.001) and the expression levels of interferon gamma and tumor necrosis factor α were significantly decreased (48.29 pg/mL ± 10.84 pg/mL vs 25.01 pg/mL ± 1.49 pg/mL, P < 0.0001) and (84.24 pg/mL ± 23.47 pg/mL vs 40.66 pg/mL ± 14.65 pg/mL, P < 0.001). The CD4+ T lymphocytes, CD8+ T lymphocytes and B lymphocytes in the liver were significantly reduced, with statistically significant differences (24.21% ± 6.55% vs 15.98% ± 3.03%, P < 0.05; 9.09% ± 1.91% vs 5.49% ± 1.00%, P < 0.001; 80.51% ± 2.96% vs 75.31% ± 4.34%, P < 0.05). The expression of CD8+ T lymphocytes and B lymphocytes in the ImmTOR treatment group also decreased (9.09% ± 1.91% vs 5.49% ± 1.00%, P < 0.001; 80.51% ± 2.96% vs 75.31% ± 4.34%, P < 0.05). The liver pathology of PBC mice in the treatment group showed reduced inflammation and a decreased total pathology score, and the difference in the scores was statistically significant (4.50 ± 2.88 vs 1.75 ± 1.28, P < 0.05).
Conclusion: ImmTOR can improve biochemistry and pathology of liver obvious by inhibiting the expression of CD8+ T cells and B cells, and reducing the titer of AMA.
Keywords: Anti-mitochondrial antibodies; Cytokine; Mouse model; Nanoparticles; Primary biliary cholangitis; Rapamycin.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors declare no relevant conflicts of interest for this article.
Figures


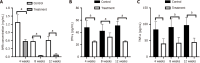

Similar articles
-
[Ascites CD100 levels and immunomodulation effects in the peripheral blood of patients with liver cirrhosis combined with spontaneous bacterial peritonitis].Zhonghua Gan Zang Bing Za Zhi. 2023 Feb 20;31(2):138-146. doi: 10.3760/cma.j.cn501113-20220314-00108. Zhonghua Gan Zang Bing Za Zhi. 2023. PMID: 37137828 Chinese.
-
[Experimental study of anti-tumor immunologic response functions after combined treatment of cryoablation with GM-CSF for colorectal cancer liver metastases].Zhonghua Nei Ke Za Zhi. 2025 Jul 1;64(7):670-674. doi: 10.3760/cma.j.cn112138-20241115-00757. Zhonghua Nei Ke Za Zhi. 2025. PMID: 40605293 Chinese.
-
[Neuroprotective Effects of Anisodine Hydromide in a Rat Model of Vascular Dementia and the Antioxidative Stress Mechanisms Involved].Sichuan Da Xue Xue Bao Yi Xue Ban. 2025 Mar 20;56(2):324-330. doi: 10.12182/20250360505. Sichuan Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40599285 Free PMC article. Chinese.
-
Bezafibrate for primary biliary cirrhosis.Cochrane Database Syst Rev. 2012 Jan 18;1(1):CD009145. doi: 10.1002/14651858.CD009145.pub2. Cochrane Database Syst Rev. 2012. PMID: 22259000 Free PMC article.
-
Ursodeoxycholic acid for primary biliary cirrhosis.Cochrane Database Syst Rev. 2012 Dec 12;12(12):CD000551. doi: 10.1002/14651858.CD000551.pub3. Cochrane Database Syst Rev. 2012. PMID: 23235576 Free PMC article.
References
-
- Lv T, Chen S, Li M, Zhang D, Kong Y, Jia J. Regional variation and temporal trend of primary biliary cholangitis epidemiology: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:1423–1434. - PubMed
-
- Faisal MS, Gonzalez HC, Gordon SC. Primary Biliary Cholangitis: Epidemiology, Diagnosis, and Presentation. Clin Liver Dis. 2024;28:63–77. - PubMed
-
- Fan GH, Zhang CZ, Gao FQ, Wei XY, Ling SB, Wang K, Wang JG, Zheng SS, Nikfarjam M, Xu X. A mixed blessing for liver transplantation patients - Rapamycin. Hepatobiliary Pancreat Dis Int. 2023;22:14–21. - PubMed
-
- Yang L, Bracho-Sanchez E, Fernando LP, Lewis JS, Carstens MR, Duvall CL, Keselowsky BG. Poly(2-propylacrylic acid)/poly(lactic-co-glycolic acid) blend microparticles as a targeted antigen delivery system to direct either CD4(+) or CD8(+) T cell activation. Bioeng Transl Med. 2017;2:202–211. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

