Sex-related differences in patients with chronic hepatitis C infection treated with direct-acting antiviral drugs
- PMID: 40606929
- PMCID: PMC12210159
- DOI: 10.4254/wjh.v17.i6.105899
Sex-related differences in patients with chronic hepatitis C infection treated with direct-acting antiviral drugs
Abstract
Background: Sex is one of the known factors influencing the risk of hepatitis C virus (HCV) infection and the natural course of the disease.
Aim: To evaluate sex-related differences in the characteristics and outcomes of direct-acting antiviral (DAA) treatment in HCV-infected patients.
Methods: The study included consecutive 9457 women and 9529 men, treated with DAA for chronic HCV infection from July 2015 to the end of 2023 whose data were collected in the nationwide multicenter retrospective Epiter-2 project. Women were divided into pre-menopausal (15-44 years), menopausal (45-55 years) and post-menopausal (> 55 years) and compared with age-matched men.
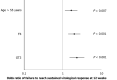
Results: Regardless of age, women had a significantly lower body mass index, prevalence of genotype 3 infection and proportion of cirrhosis compared to men. Psychiatric disorders (except depression), hepatitis B virus and human immunodeficiency virus co-infections, as well as alcohol and drug addiction, were significantly less common in women than in men in all age groups. The sustained virologic response was significantly higher in women compared to men in each age group and amounted to 98.4% and 96.6%, respectively (P < 0.001). Independent predictors of treatment failure in women were genotype 3 infection, cirrhosis and postmenopausal age. Mild adverse events were reported significantly more often by women, regardless of age with the highest percentage in the postmenopausal group.
Conclusion: DAA treatment is more effective in women than in men, regardless of age, but in postmenopausal women, the effectiveness is relatively the lowest.
Keywords: Chronic hepatitis C; Direct-acting antivirals; Hepatitis C virus; Menopause; Women.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Zarębska-Michaluk D has acted as a speaker and advisor for AbbVie and Gilead. Pawłowska M has acted as a speaker for AbbVie and Gilead. Berak H has acted as a speaker for Abbvie. Janczewska E has acted as a speaker and/or advisor for AbbVie, Gilead, MSD, and Ipsen, and has received funding for clinical trials from AbbVie, Allergan, BMS, Celgene, Cymabay, Dr Falk Pharma, Exelixis, GSK, and MSD. Mazur W has acted as a speaker and/or advisor for AbbVie, Gilead, and Merck, and has received funding for clinical trials from AbbVie, Gilead, and Janssen. Klapaczyński J has acted as a speaker for Gilead and AbbVie. Sitko M has acted as a speaker for AbbVie and Gilead. Piekarska A has acted as a speaker and/or advisor for AbbVie, Gilead, Merck, and Roche; Jaroszewicz J has acted as a speaker and/or advisor for AbbVie, Gilead, Merck, Roche, Alfasigma, MSD, Gilead, and PRO.MED. Flisiak R has acted as a speaker and/or advisor and has received funding for clinical research from AbbVie, Gilead, Merck, Roche, and Novo Nordisk. Dobrowolska K, Tudrujek-Zdunek M, Janocha-Litwin J, and Dybowska D have no conflict to declare.
Figures

Similar articles
-
Direct-acting antivirals for chronic hepatitis C.Cochrane Database Syst Rev. 2017 Sep 18;9(9):CD012143. doi: 10.1002/14651858.CD012143.pub3. Cochrane Database Syst Rev. 2017. PMID: 28922704 Free PMC article.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
Pharmacological interventions for acute hepatitis C infection: an attempted network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 13;3(3):CD011644. doi: 10.1002/14651858.CD011644.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2018 Dec 03;12:CD011644. doi: 10.1002/14651858.CD011644.pub3. PMID: 28285495 Free PMC article. Updated.
-
Direct-acting antivirals for chronic hepatitis C.Cochrane Database Syst Rev. 2017 Jun 6;6(6):CD012143. doi: 10.1002/14651858.CD012143.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2017 Sep 18;9:CD012143. doi: 10.1002/14651858.CD012143.pub3. PMID: 28585310 Free PMC article. Updated.
-
NIH Consensus Statement on Management of Hepatitis C: 2002.NIH Consens State Sci Statements. 2002 Jun 10-12;19(3):1-46. NIH Consens State Sci Statements. 2002. PMID: 14768714
References
-
- Baden R, Rockstroh JK, Buti M. Natural history and management of hepatitis C: does sex play a role? J Infect Dis. 2014;209 Suppl 3:S81–S85. - PubMed
-
- Simões D, Stengaard AR, Combs L, Raben D EuroTEST COVID-19 impact assessment consortium of partners. Impact of the COVID-19 pandemic on testing services for HIV, viral hepatitis and sexually transmitted infections in the WHO European Region, March to August 2020. Euro Surveill. 2020;25:2001943. - PMC - PubMed
LinkOut - more resources
Full Text Sources

