The efficacy of infliximab combined with partial enteral nutrition in the treatment of Crohn's disease: a cohort study
- PMID: 40607024
- PMCID: PMC12218248
- DOI: 10.3389/fnut.2025.1591954
The efficacy of infliximab combined with partial enteral nutrition in the treatment of Crohn's disease: a cohort study
Abstract
Background and aims: The issue of loss of efficacy with infliximab (IFX) treatment in Crohn's disease (CD) significantly limits its clinical use. This study aims to investigate the role of therapy combined with partial enteral nutrition (PEN) in maintaining the efficacy of infliximab.
Methods: Consecutive CD patients undergoing IFX for induction and maintenance therapy were included, with a follow-up period of at least 54 weeks and endoscopy performed around 54 weeks. Subsequent longitudinal monitoring evaluated improvements in the Crohn's Disease Activity Index (CDAI) score at 14 weeks and endoscopic remission at 54 weeks.
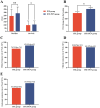
Results: Among the 176 included patients, 99 (56%) were in the IFX monotherapy group, and 77 (44%) were in the IFX + PEN group. A significantly higher proportion of patients in the IFX + PEN group achieved clinical response (defined as a CDAI decrease ≥70 points) compared to those in the IFX group at 14 weeks (87.01% vs. 74.75%, p = 0.043), as well as a higher proportion achieving endoscopic remission at 54 weeks (84.42% vs. 65.66%, p = 0.005). Meanwhile, combination therapy with PEN emerged as an independent protective predictor of endoscopic remission at 54 weeks in two multivariate-adjusted models, with ORs of 3.34 and 3.33, respectively (both p < 0.05). Subgroup analysis and interaction test results further supported that all CD patients can benefit from combination therapy with PEN.
Conclusion: Infliximab treatment combined with partial enteral nutrition is beneficial for both short-term clinical response and long-term endoscopic remission in CD patients.
Keywords: Crohn’s disease; clinical response; endoscopic remission; infliximab; partial enteral nutrition; safety.
Copyright © 2025 Huang, Chen, Wu, Yin, Yao, Bai, Zhuo and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A systematic review and economic evaluation of the use of tumour necrosis factor-alpha (TNF-α) inhibitors, adalimumab and infliximab, for Crohn's disease.Health Technol Assess. 2011 Feb;15(6):1-244. doi: 10.3310/hta15060. Health Technol Assess. 2011. PMID: 21291629 Free PMC article.
-
Low dose naltrexone for induction of remission in Crohn's disease.Cochrane Database Syst Rev. 2018 Apr 1;4(4):CD010410. doi: 10.1002/14651858.CD010410.pub3. Cochrane Database Syst Rev. 2018. PMID: 29607497 Free PMC article.
-
Withdrawal of immunosuppressant or biologic therapy for patients with quiescent Crohn's disease.Cochrane Database Syst Rev. 2018 May 12;5(5):CD012540. doi: 10.1002/14651858.CD012540.pub2. Cochrane Database Syst Rev. 2018. PMID: 29756637 Free PMC article.
-
Enteral nutritional therapy for induction of remission in Crohn's disease.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD000542. doi: 10.1002/14651858.CD000542.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2018 Apr 01;4:CD000542. doi: 10.1002/14651858.CD000542.pub3. PMID: 17253452 Updated.
-
Enteral nutritional therapy for inducing remission of Crohn's disease.Cochrane Database Syst Rev. 2001;(3):CD000542. doi: 10.1002/14651858.CD000542. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2007 Jan 24;(1):CD000542. doi: 10.1002/14651858.CD000542.pub2. PMID: 11686966 Updated.
References
-
- Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. (2021) 160:1570–83. doi: 10.1053/j.gastro.2020.12.031, PMID: - DOI - PubMed
-
- Hazlewood GS, Rezaie A, Borman M, Panaccione R, Ghosh S, Seow CH, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn's disease: a network meta-analysis. Gastroenterology. (2015) 148:344–354.e5. doi: 10.1053/j.gastro.2014.10.011, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

