Resonsive anion transport with a Hamilton receptor-based anionophore controlled by photo-activation and host-guest competitive inhibition
- PMID: 40607051
- PMCID: PMC12211230
- DOI: 10.1039/d5sc02619a
Resonsive anion transport with a Hamilton receptor-based anionophore controlled by photo-activation and host-guest competitive inhibition
Abstract
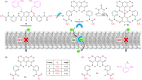
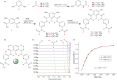
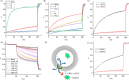
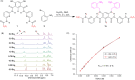
Ion transport across biological membranes, facilitated by naturally occurring ion channels and pumps, is crucial for many biological functions. Many of these transport systems are gated, such that ion transport is regulated by a range of external stimuli, including light, small molecule ligand binding, and membrane potential. Synthetic ion transport systems, including those with similar gating mechanisms, have garnered significant attention due to their potential applications in targeted therapeutics as anticancer agents or to treat channelopathies. In this work, we report stimuli-responsive anion transporters based on dynamic hydrogen bonding interactions of hydroxyl-functionalised Hamilton-receptor-based anionophores. Caging of the hydroxyl groups with a light-responsive ortho-nitrobenzyl (ONB) moiety locks the amide protons through intramolecular hydrogen bonding, making them unavailable for anion binding and transport. Decaging with light reverses the hydrogen bonding pattern, rendering the amide protons available for anion binding and transport. Addition of a barbiturate ligand switches OFF the ion transport activity by blocking the anion binding cavity through competitive inhibition. OFF-ON-OFF reversible control over anion transport is therefore achieved using a combination of light and competitive small molecular ligand binding.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Stimuli-responsive anion transport utilising caged hydrazone-based anionophores.Nanoscale. 2024 Nov 28;16(46):21545-21553. doi: 10.1039/d4nr03220a. Nanoscale. 2024. PMID: 39480659
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K. and Walter P., Molecular Biology of the Cell, 4th edn, 2002
-
- Moore S. J. Haynes C. J. E. González J. Sutton J. L. Brooks S. J. Light M. E. Herniman J. Langley G. J. Soto-Cerrato V. Pérez-Tomás R. Marques I. Costa P. J. Félix V. Gale P. A. Chem. Sci. 2013;4:103–117. doi: 10.1039/C2SC21112B. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

