Bidirectional mechanisms and emerging strategies for implantable bioelectronic interfaces
- PMID: 40607118
- PMCID: PMC12221386
- DOI: 10.1016/j.bioactmat.2025.06.014
Bidirectional mechanisms and emerging strategies for implantable bioelectronic interfaces
Abstract
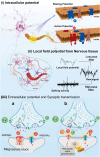
Neural network functionality depends on the signaling of excitable cells and intricate synaptic connections, which collectively promote advanced functions of the brain, such as perception, motor control, and cognition. Neurological diseases may cause changes in the structure and connection patterns of neural networks, thereby leading to loss of motor and sensory functions. Neural interfaces are dependable tools for recording or stimulating neural circuit dynamics, but conventional neural implants do not align with the physicochemical characteristics of living tissues, resulting in eventual failure of these interface devices. These challenges in neuroengineering have spurred progress in materials science. In this account, we explore the interaction mechanisms between electrodes and biological tissues, offering strategies to meet the electrochemical and biocompatibility demands of bioelectronic interfaces in engineering, with an emphasis on the structural design and manufacturing technologies of implantable devices.
Keywords: Nanostructures; Neural implantable device; Neural interface; Neural probe design.
© 2025 The Authors.
Conflict of interest statement
The authors declare the following personal relationships which may be considered as potential competing interests: Changmao Ni and Li Huang are currently employed by Wuhan Neuracom Technology Development Co., Ltd.
Figures










Similar articles
-
Revolutionizing brain‒computer interfaces: overcoming biocompatibility challenges in implantable neural interfaces.J Nanobiotechnology. 2025 Jul 10;23(1):498. doi: 10.1186/s12951-025-03573-x. J Nanobiotechnology. 2025. PMID: 40640801 Free PMC article. Review.
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
The health economics of insulin therapy: How do we address the rising demands, costs, inequalities and barriers to achieving optimal outcomes.Diabetes Obes Metab. 2025 Jul;27 Suppl 5(Suppl 5):24-35. doi: 10.1111/dom.16488. Epub 2025 Jun 4. Diabetes Obes Metab. 2025. PMID: 40464081 Free PMC article.
References
-
- Stuart G., Spruston N., Sakmann B., Hausser M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 1997;20(3):125–131. - PubMed
-
- Bejot Y., Bailly H., Durier J., Giroud M. Epidemiology of stroke in Europe and trends for the 21st century. Presse Med. 2016;45(12 Pt 2):e391–e398. - PubMed
-
- Calabrese V.P. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;69(2):223–224. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

