Development and implementation of a TaqMan triplex real-time PCR assay for concurrent detection of pseudorabies virus, porcine teschovirus 1, and Streptococcus suis 2
- PMID: 40607354
- PMCID: PMC12213347
- DOI: 10.3389/fvets.2025.1589175
Development and implementation of a TaqMan triplex real-time PCR assay for concurrent detection of pseudorabies virus, porcine teschovirus 1, and Streptococcus suis 2
Abstract
Introduction: Porcine neurological disorders represent a prevalent clinical condition that leads to significant mortality and economic losses within the swine industry. Pseudorabies virus (PRV), porcine teschovirus 1 (PTV1), and Streptococcus suis 2 (SS2) are key viral and bacterial pathogens implicated in the manifestation of neurological symptoms in pig populations. The overlapping clinical presentations and pathological alterations associated with these pathogens pose challenges in their clinical differentiation. Therefore, it is essential to develop a diagnostic method with high sensitivity and specificity that can simultaneously detect and differentiate these viral and bacterial agents.
Materials and methods: A triplex real-time PCR assay using TaqMan probes was developed to simultaneously detect PRV, PTV1, and SS2. To assess the efficacy of the established assay, 30 clinical samples of animals with nervous symptoms were used to compare the results obtained from the triplex real-time PCR assay with those obtained from commercial singleplex real-time PCR kits. Furthermore, a total of 282 samples were tested and analyzed to validate the utility of the assay.
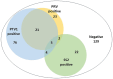
Results: The triplex real-time PCR assay exhibited high sensitivity, specificity, and repeatability, with a detection limit of 1.0 × 100 copy/μL. The triplex real-time PCR method and commercial singleplex real-time PCR kits showed complete concordance in detecting PRV, PTV1, and SS2. Clinical data indicated single infection rates of 8.16% for PRV, 26.95% for PTV1, and 7.80% for SS2. The observed co-infection rates were 7.45% for PRV + PTV1, 0.71% for PRV + SS2, 1.42% for PTV1 + SS2, and 1.77% for PRV + PTV1 + SS2, respectively.
Conclusion: The triplex real-time PCR method developed in this study effectively distinguishes PRV, PTV1, and SS2 simultaneously, serving as a valuable diagnostic tool. This method is anticipated to play a crucial role in preventing and controlling infectious disease spread and supporting epidemiological investigations.
Keywords: PRV; PTV1; SS2; porcine nervous diseases; triplex real-time PCR.
Copyright © 2025 Lai, Yang, Wu, Wu, Li, Liu, Yan, Yu, Ren, Hu and Li.
Conflict of interest statement
RL, CY, LW, SR, and XL were employed by the Shandong New Hope Liuhe Group Co., Ltd. RL, CY, LW, LL, ZY, and XL were employed by the Juye Xinhao Agriculture and Animal Husbandry Co., Ltd. WW, WL, DY, and XL were employed by the New Hope Liuhe Co., Ltd. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Development and Application of a Multiplex Real-Time TaqMan qPCR Assay for the Simultaneous Detection of African Swine Fever Virus, Classical Swine Fever Virus, Porcine Reproductive and Respiratory Syndrome Virus, Pseudorabies Virus, and Porcine Circovirus Type 2.Microorganisms. 2025 Jul 3;13(7):1573. doi: 10.3390/microorganisms13071573. Microorganisms. 2025. PMID: 40732082 Free PMC article.
-
Performance evaluation of TaqMan™ Arbovirus Triplex Kit (ZIKV/DENV/CHIKV) for detection and differentiation of dengue and chikungunya viral RNA in serum samples of symptomatic patients.J Virol Methods. 2025 Feb;332:115072. doi: 10.1016/j.jviromet.2024.115072. Epub 2024 Nov 20. J Virol Methods. 2025. PMID: 39571938
-
Assessment of a multiplex arbovirus PCR Detection Test in an area endemic for Chikungunya, Zika, and Dengue viruses: An evaluation of kit performance characteristics in line with Clinical Laboratory Improvement Amendments (CLIA) Standards.PLoS One. 2025 Jun 24;20(6):e0309626. doi: 10.1371/journal.pone.0309626. eCollection 2025. PLoS One. 2025. PMID: 40554518 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Ko CC, Merodio MM, Spronk E, Lehman JR, Shen H, Li G, et al. Diagnostic investigation of Mycoplasma hyorhinis as a potential pathogen associated with neurological clinical signs and central nervous system lesions in pigs. Microb Pathog. (2023) 180:106172. doi: 10.1016/j.micpath.2023.106172, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

