Revisiting pulmonary fibrosis: inflammatory dynamics of the lipofibroblast-to-inflammatory lipofibroblast-to-activated myofibroblast reversible switch
- PMID: 40607394
- PMCID: PMC12213413
- DOI: 10.3389/fimmu.2025.1609509
Revisiting pulmonary fibrosis: inflammatory dynamics of the lipofibroblast-to-inflammatory lipofibroblast-to-activated myofibroblast reversible switch
Abstract
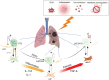
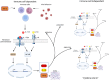
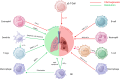
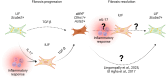
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive interstitial lung disease characterized by excessive extracellular matrix (ECM) deposition and irreversible lung damage. A key driver of disease progression is the phenotypic shift of lipofibroblasts (LIFs) into activated myofibroblasts (aMYFs), triggered by sustained epithelial injury, caused by inflammation, oxidative stress, viral infections (e.g., influenza, SARS-CoV-2), and metabolic dysfunction. Emerging evidence demonstrates that this transition is reversible, with pharmacological agents that promote aMYF-to-LIF reprogramming contributing to fibrosis resolution. The identification of inflammatory lipofibroblasts (iLIFs) highlights the importance of inflammation in fibrosis progression. Inflammation, mediated by IL-1β, IL-17A, and TGF- β, sustain aMYF activation, while immune cells shape fibrosis formation. This review combines current insights on the cellular and molecular pathways controlling fibroblast differentiation, highlighting key metabolic, immunologic, and oxidative stress-modulating targets for therapeutic intervention. Understanding and manipulating the LIF-iLIF-aMYF axis offers a promising strategy for reversing fibrosis and restoring pulmonary homeostasis in IPF.
Keywords: IL-17A; TGF-β; activated myofibroblast; idiopathic pulmonary fibrosis; inflammation; inflammatory lipofibroblast; lipofibroblast; virus infection.
Copyright © 2025 Panagiotidis, Vasquez-Pacheco, Chu, Seeger, El Agha, Bellusci and Lingampally.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

