Colorectal cancer-infiltrating NK cell landscape analysis unravels tissue-resident PD-1+ NK cells in microsatellite instability tumors
- PMID: 40607422
- PMCID: PMC12213648
- DOI: 10.3389/fimmu.2025.1578444
Colorectal cancer-infiltrating NK cell landscape analysis unravels tissue-resident PD-1+ NK cells in microsatellite instability tumors
Abstract
Background: Natural killer (NK) cells are innate lymphocytes endowed with potent cytotoxic activity. The presence of tumor-associated NK cells has been correlated with better prognosis in several solid tumors including colorectal cancer (CRC). This malignant disease is the second cause of cancer death worldwide and is in urgent need for novel approaches to improve current immunotherapies. Since CRC microenvironment can induce NK cell dysfunction and hinder cancer control, understanding tumor-associated NK cell features is mandatory to fully unlock their immunotherapeutic potential.
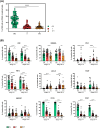
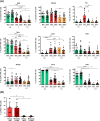
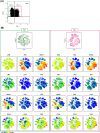
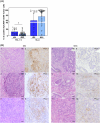
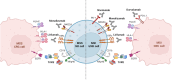
Purpose: Our study aims at elucidating the molecular and functional characteristics of tumor-associated NK cells in CRC focusing on the expression of immune checkpoints that critically regulate NK cell function. We performed an in-depth cytofluorimetric analysis of tumor-associated NK cells obtained by tissue dissociation of samples derived from 80 CRC patients comparing tumor with matched tumor-free tissue and peripheral blood, stratifying patients by tumor stage or MSI/MSS condition. Tumor tissue was also analyzed by immunohistochemistry.
Results: NK cells expressing immune checkpoints (i.e., KIR, NKG2A and TIM-3) were significantly enriched in tumor compared to tumor-free tissue, and an increase in PD-1+ NK cells was observed in tumors compared to peripheral blood and tumor-free tissue, indicating TME-induced modulation. Notably, tumor-associated PD-1+ NK cells characterized MSI rather than MSS CRC. In addition, tumor-associated NK cells also expressed tissue residency markers (CD103 and/or CD49a) and displayed a distinct profile also including the PD-1+ NK cell subset in MSI CRC, possibly representing NK cells recruited from circulation, retained in tumors, and reconfigured by TME signals. Importantly, tissue resident NK cells adequately expressed activating NK receptors and cytotoxic molecules.
Conclusions: These results suggest, together with an increased PD-L1 expression on MSI tumor cells, that the efficacy of immunotherapies in MSI CRC based on PD-1/PD-L1 blockade could also rely on a superior anti-tumor potential of PD-1+ NK cells. Conversely, MSS CRC, in which tumor-associated PD-1+ NK cells are scarce, could benefit more from immunotherapies blocking NKG2A and/or KIRs. Thus, novel approaches based on NK cell features related to CRC type, fully exploiting circulating and resident NK cell anti-tumor activity, could be key to next-generation therapies.
Keywords: colorectal cancer; human NK cells; immune checkpoints; microsatellite status; tissue-residency markers.
Copyright © 2025 Obino, Giordano, Carlomagno, Setti, Greppi, Bozzo, Pesce, Ferretti, Candiani, Muccio, Ciferri, Buttiron Webber, Solari, Ortolani, Paleari, Clavarezza, Barberis, Filauro, Provinciali, Rutigliani, Marcenaro, DeCensi, Della Chiesa and Sivori.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

