Chemical Composition and Backbone Modifications Define Deformability of Nucleic Acid Nanoparticles
- PMID: 40607511
- PMCID: PMC12269358
- DOI: 10.1021/acsnano.5c04293
Chemical Composition and Backbone Modifications Define Deformability of Nucleic Acid Nanoparticles
Abstract
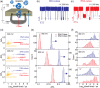
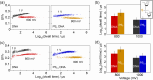
Nucleic acid nanoparticles (NANPs), composed of short oligonucleotides assembled into specific architectures, are emerging as a programmable platform for the regulated drug delivery of various therapeutic agents. Here, we use a nanopore "clamp" to investigate the mechanical properties of six-stranded RNA and DNA-based NANPs with the connectivity of a cube of sizes below 10 nm. When electrophoretically forced through solid-state nanopores that are smaller than the cubes, deformation of the NANPs generates prolonged electrical signatures whose durations depend on the mechanical deformability of the structures. All-atom MD simulations further reveal differences in the mechanical flexibility of DNA, RNA, modified RNA, and hybrid DNA/RNA cubes, supporting these findings at the molecular level. While DNA cubes deform and translocate through the pore, analogous RNA cubes are too stiff and cannot squeeze through at a comparable voltage, despite having the same sequence and overall shape as the DNA cubes. Further, we find that hybrid RNA/DNA cubes exhibit intermediate mechanical deformability to pure DNA or RNA cubes, indicating an additive effect of the RNA content on nanocube stiffness. Finally, different chemical modifications introduced to the strands can be used to fine-tune the mechanical properties of the NANPs.
Keywords: MD simulations; flexibility; nucleic acid nanoparticles; solid-state nanopore; translocation.
Figures




Similar articles
-
The immunorecognition, subcellular compartmentalization, and physicochemical properties of nucleic acid nanoparticles can be controlled by composition modification.Nucleic Acids Res. 2020 Nov 18;48(20):11785-11798. doi: 10.1093/nar/gkaa908. Nucleic Acids Res. 2020. PMID: 33091133 Free PMC article.
-
Nucleic Acid Nanocapsules as a New Platform to Deliver Therapeutic Nucleic Acids for Gene Regulation.Acc Chem Res. 2025 Jul 1;58(13):1951-1962. doi: 10.1021/acs.accounts.5c00126. Epub 2025 Jun 9. Acc Chem Res. 2025. PMID: 40491030
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
-
Ventilator Management(Archived).2023 Mar 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 Mar 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 28846232 Free Books & Documents.
-
Factors that impact on the use of mechanical ventilation weaning protocols in critically ill adults and children: a qualitative evidence-synthesis.Cochrane Database Syst Rev. 2016 Oct 4;10(10):CD011812. doi: 10.1002/14651858.CD011812.pub2. Cochrane Database Syst Rev. 2016. PMID: 27699783 Free PMC article.
References
-
- Manrao E. A., Derrington I. M., Laszlo A. H., Langford K. W., Hopper M. K., Gillgren N., Pavlenok M., Niederweis M., Gundlach J. H.. Reading DNA at single-nucleotide resolution with a mutant MspA nanopore and phi29 DNA polymerase. Nat. Biotechnol. 2012;30(4):349–353. doi: 10.1038/nbt.2171. - DOI - PMC - PubMed
-
- Sutherland T. C., Long Y.-T., Stefureac R.-I., Bediako-Amoa I., Kraatz H.-B., Lee J. S.. Structure of Peptides Investigated by Nanopore Analysis. Nano Lett. 2004;4(7):1273–1277. doi: 10.1021/nl049413e. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

