The Role of Iron Chelation Therapy in Colorectal Cancer: A Systematic Review on Its Mechanisms and Therapeutic Potential
- PMID: 40607631
- PMCID: PMC12224056
- DOI: 10.1002/cam4.71019
The Role of Iron Chelation Therapy in Colorectal Cancer: A Systematic Review on Its Mechanisms and Therapeutic Potential
Abstract
Background: Despite significant therapeutic advancements in recent decades, colorectal cancer (CRC) continues to exhibit high rates of mortality and morbidity. Chemoresistance and cancer recurrence remain substantial challenges, underscoring the need for novel treatment approaches. Iron chelation therapy has gained profound interest over the years as a potential cancer treatment, leveraging the increased iron demand by tumors. This review evaluates the effects of iron chelation therapy on CRC progression and the underlying mechanisms.
Method: A comprehensive review of in vivo and in vitro studies was conducted to assess the effectiveness of iron chelation therapy in CRC. The literature search covered PubMed, Scopus, Medline (via Web of Science), and EMBASE between January 1995 and March 2024.
Results: Several in vitro and in vivo studies have investigated the impact of iron chelators, such as deferoxamine, deferasirox, thiosemicarbazone-based chelators, quilamine-based chelators, and other novel compounds on CRC. Natural plant extracts with iron-chelating properties have also been explored as potential treatments. Most studies indicate that iron chelation can inhibit the proliferation of colon cancer cells, though some studies suggest cancer-promoting effects. Mechanistically, iron chelation affects several hallmarks of CRC by modulating histone methylation, upregulating NDRG1, and influencing the Wnt/β-catenin and p53 signaling pathways. However, certain iron chelators may inhibit TRAIL-mediated apoptosis and activate the hypoxia-inducible factor (HIF), potentially accelerating CRC progression.
Conclusion: Future exploration of iron chelation therapy in CRC should focus on extensive in vitro, in vivo, and clinical studies to elucidate the precise mechanisms involved. A deeper understanding of the genetic and cellular alterations induced by iron chelation will enhance the development of effective therapeutic strategies for CRC.
Keywords: Colorectal cancer; anticancer effects; iron chelation; systematic review.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
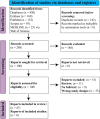
Figures



Similar articles
-
Interventions for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia.Cochrane Database Syst Rev. 2018 May 8;5(5):CD012349. doi: 10.1002/14651858.CD012349.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2023 Mar 6;3:CD012349. doi: 10.1002/14651858.CD012349.pub3. PMID: 29737522 Free PMC article. Updated.
-
Desferrioxamine mesylate for managing transfusional iron overload in people with transfusion-dependent thalassaemia.Cochrane Database Syst Rev. 2013 Aug 21;2013(8):CD004450. doi: 10.1002/14651858.CD004450.pub3. Cochrane Database Syst Rev. 2013. PMID: 23963793 Free PMC article.
-
Deferasirox for managing iron overload in people with thalassaemia.Cochrane Database Syst Rev. 2017 Aug 15;8(8):CD007476. doi: 10.1002/14651858.CD007476.pub3. Cochrane Database Syst Rev. 2017. PMID: 28809446 Free PMC article.
-
Deferasirox for managing iron overload in people with thalassaemia.Cochrane Database Syst Rev. 2012 Feb 15;(2):CD007476. doi: 10.1002/14651858.CD007476.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Aug 15;8:CD007476. doi: 10.1002/14651858.CD007476.pub3. PMID: 22336831 Updated.
-
Oral deferiprone for iron chelation in people with thalassaemia.Cochrane Database Syst Rev. 2013 Aug 21;2013(8):CD004839. doi: 10.1002/14651858.CD004839.pub3. Cochrane Database Syst Rev. 2013. PMID: 23966105 Free PMC article.
References
-
- WHO , “Colorectal Cancer,” 2023, https://www.who.int/news‐room/fact‐sheets/detail/colorectal‐cancer.
-
- Holleczek B., Rossi S., Domenic A., et al., “On‐Going Improvement and Persistent Differences in the Survival for Patients With Colon and Rectum Cancer Across Europe 1999‐2007—Results From the EUROCARE‐5 Study,” European Journal of Cancer 51, no. 15 (2015): 2158–2168, 10.1016/j.ejca.2015.07.024. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

