IFNγ-inducible Gbp4 and Irgb6 contribute to experimental cerebral malaria pathology in the olfactory bulb
- PMID: 40607809
- PMCID: PMC12345229
- DOI: 10.1128/mbio.01249-25
IFNγ-inducible Gbp4 and Irgb6 contribute to experimental cerebral malaria pathology in the olfactory bulb
Abstract
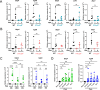
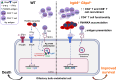
Cerebral malaria (CM) is a severe and often fatal complication of Plasmodium falciparum infection. Although much progress has been made in understanding CM, the precise pathogenesis remains elusive. The olfactory bulb (OB) has emerged as a critical site of immunopathology in experimental cerebral malaria (ECM) models, but its contribution to disease progression is not fully understood. To investigate the molecular mechanisms driving early ECM pathogenesis, we conducted transcriptomic profiling of the OB to identify key genes associated with disease onset. Our analysis revealed significant early upregulation of interferon (IFN)-inducible GTPases, particularly Irgb6 and Gbp4, effectors downstream of IFN-γ but not IFN-α/β signaling, suggesting their involvement in ECM pathology. Using Gbp4-/-, Irgb6-/-, and double knockout (Irgb6-/- Gbp4-/-) mice, we identified a pathological role for these GTPases. Mechanistically, we found that double-knockout mice exhibited increased infiltration of CD4+ and CD8+ T cells into the brain but with reduced T cell functionality and impaired antigen presentation by endothelial cells, leading to enhanced parasite accumulation in the OB. This disruption in immune regulation ultimately conferred improved survival in the Irgb6-/- Gbp4-/- mice and indicated the pathological impact of Gbp4 and Irgb6 in ECM. These findings reveal that Gbp4 and Irgb6 play important roles in the early immunopathogenesis of ECM by modulating antigen processing and presentation in the OB, thereby shaping immune cell dynamics. Our work shows the dual role of Irgb6 and Gbp4 GTPases in host defence and immunopathology and offers new insights into ECM mechanisms and antigen presentation.IMPORTANCECerebral malaria (CM) arises from an excessive inflammatory response and blood-brain-barrier (BBB) dysfunction in Plasmodium-infected hosts, but the precise mechanisms driving early-stage pathogenesis remain unclear. Through RNA sequencing of the olfactory bulb (OB) in a murine experimental cerebral malaria (ECM) model, we identified the early upregulation of interferon (IFN)-inducible GTPases, Irgb6 and Gbp4, key effectors downstream of IFN-γ signaling. Our results demonstrate that Gbp4 and Irgb6 synergistically contribute to ECM pathology by regulating antigen cross-presentation in endothelial cells. This dysregulation leads to abnormal parasite burden and alters the accumulation of CD4+ and CD8+ T cells in the brain via the OB, further perturbing inflammation. Our findings suggest a novel mechanism in CM and emphasize the pivotal roles of Gbp4 and Irgb6 in promoting cell-autonomous immune responses that, in turn, escalate pathological inflammation. Our study offers insights into how dysregulated immune responses drive CM progression and suggests potential therapeutic targets to mitigate fatal outcomes.
Keywords: GTPases; Gbp4; Irgb6; cerebral malaria; olfactory bulb.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- World Health Organization (WHO). 2024. Malaria Report 2024
-
- Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, Birbeck GL, Bradley WG, Fox LL, Glover SJ, Hammond CA, Heyderman RS, Chilingulo CA, Molyneux ME, Taylor TE. 2015. Brain swelling and death in children with cerebral malaria. N Engl J Med 372:1126–1137. doi: 10.1056/NEJMoa1400116 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

