Weissella cibaria suppresses colitis-associated colorectal cancer by modulating the gut microbiota-bile acid-FXR axis
- PMID: 40607831
- PMCID: PMC12282153
- DOI: 10.1128/msystems.00288-25
Weissella cibaria suppresses colitis-associated colorectal cancer by modulating the gut microbiota-bile acid-FXR axis
Abstract
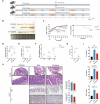
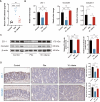
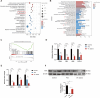
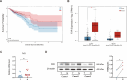
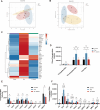
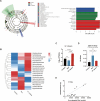
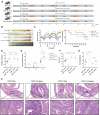
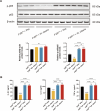
Gut microbiota dysbiosis critically contributes to colitis-associated colorectal cancer (CAC) pathogenesis, positioning microbial modulation as a promising therapeutic strategy. Weissella cibaria (W. cibaria) is an emerging probiotic with potential cancer-inhibiting effects. This study investigates the anti-tumorigenic potential of W. cibaria in an azoxymethane/dextran sulfate sodium-induced CAC murine model. Mice were orally administered W. cibaria every 2 days from the beginning of the model construction until the end of the experiment. The study demonstrated significant changes in the gut microbiota of CAC mice, with a significant increase in the relative abundance of Lactobacillaceae. Supplementation with W. cibaria restored the intestinal barrier and significantly reduced the relative abundance of Lactobacillaceae in the gut microbiota. The changes in the gut microbiota reduced bile salt hydrolase activity and unconjugated bile acid (BA), reversing tumorigenesis in CAC mice. Changes in intestinal BA after W. cibaria supplementation upregulated farnesoid X receptor (FXR) expression in the intestine of CAC mice and inhibited the nuclear factor kappa-B pathway. Our findings establish that W. cibaria mitigates CAC progression through the gut microbiota-BA-FXR axis, providing mechanistic evidence for its probiotic application in CAC prevention and therapy.IMPORTANCEChronic gut inflammation driven by microbiota dysbiosis is a pivotal contributor to colitis-associated colorectal cancer (CAC) pathogenesis. Emerging evidence highlights Weissella cibaria (W. cibaria) as a promising anti-colorectal cancer agent (Y. Du, L. Liu, W. Yan, Y. Li, et al., Sci Rep 13:21117, 2023, https://doi.org/10.1038/s41598-023-47943-7; S. Ahmed, S. Singh, V. Singh, K. D. Roberts, et al., Microorganisms 10:2427, 2022, https://doi.org/10.3390/microorganisms10122427), yet its role in CAC remains unexplored. To address this gap, we investigated the inhibitory effects of W. cibaria on CAC development in vivo and elucidated its underlying mechanisms. Our results demonstrated that oral administration of W. cibaria significantly reshaped gut microbial communities and activated bile acid (BA)-related metabolic pathways. Subsequent mechanistic studies revealed that microbiota remodeling by W. cibaria altered intestinal BA composition, particularly activating the farnesoid X receptor (FXR). FXR activation mediated by these BA shifts was identified as a critical suppressor of tumorigenesis, establishing W. cibaria as a novel probiotic capable of attenuating CAC progression. Collectively, this study uncovers a protective axis linking W. cibaria-driven microbiota modulation, BA metabolism change, and FXR-dependent tumor suppression, providing experimental evidence for probiotic-based CAC intervention strategies.
Keywords: FXR; Weissella cibaria; bile acid; colitis-associated colorectal cancer; gut microbiota.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The pasteurized Weissella cibaria alleviates sepsis-induced acute lung injury by modulation of intestinal mucus barrier and gut microbiota.J Transl Med. 2025 Jun 17;23(1):661. doi: 10.1186/s12967-025-06674-1. J Transl Med. 2025. PMID: 40528192 Free PMC article.
-
3-O-Acetyl-11-Keto-β-Boswellic Acid Suppresses Colitis-Associated Colorectal Cancer by Inhibiting the NF-Kb Signaling Pathway and Remodeling Gut Microbiota.Oncol Res. 2025 Jul 18;33(8):1969-1989. doi: 10.32604/or.2025.062386. eCollection 2025. Oncol Res. 2025. PMID: 40746878 Free PMC article.
-
Intestinal inflammation and microbiota modulation impact cochlear function: emerging insights in gut-ear axis.Cell Commun Signal. 2025 Jul 26;23(1):357. doi: 10.1186/s12964-025-02338-1. Cell Commun Signal. 2025. PMID: 40713718 Free PMC article.
-
The gut microbiota-bile acid axis: a crucial regulator of immune function and metabolic health.World J Microbiol Biotechnol. 2025 Jun 25;41(7):215. doi: 10.1007/s11274-025-04395-7. World J Microbiol Biotechnol. 2025. PMID: 40555888 Review.
-
Synbiotics, prebiotics and probiotics for people with chronic kidney disease.Cochrane Database Syst Rev. 2023 Oct 23;10(10):CD013631. doi: 10.1002/14651858.CD013631.pub2. Cochrane Database Syst Rev. 2023. PMID: 37870148 Free PMC article.
References
-
- Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, Xiao X, Kwong TNY, Tsoi H, Wu WKK, Zeng B, Chan FKL, Sung JJY, Wei H, Yu J. 2017. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology 153:1621–1633. doi: 10.1053/j.gastro.2017.08.022 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
