Unraveling Relatlimab-Specific Biology Using Biomarker Analyses in Patients with Advanced Melanoma in RELATIVITY-047
- PMID: 40607902
- PMCID: PMC12402777
- DOI: 10.1158/1078-0432.CCR-24-2499
Unraveling Relatlimab-Specific Biology Using Biomarker Analyses in Patients with Advanced Melanoma in RELATIVITY-047
Abstract
Purpose: Administration of the lymphocyte activation gene 3 (LAG-3) inhibitor relatlimab (RELA) and the PD-1 inhibitor nivolumab (NIVO) significantly prolonged progression-free survival (PFS) versus NIVO alone in patients with advanced melanoma treated in RELATIVITY-047. This report describes correlative analyses of biospecimens collected within that trial to better understand the mechanisms of action and identify patients who could benefit from treatment with NIVO + RELA.
Patients and methods: Pre- and on-treatment peripheral blood samples from 563 patients were analyzed using flow cytometry for changes in 77 prespecified immune cell populations and using immunoassay for peripheral IFNγ. Pretreatment tumor biopsies were evaluated using IHC and RNA sequencing.
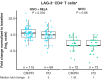
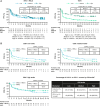
Results: On-treatment expansion of 25 peripheral immune cell populations was significantly greater with NIVO + RELA versus NIVO alone. Responders to NIVO + RELA had greater on-treatment increases in LAG-3+CD4+ T cells than nonresponders. Significantly greater increases in peripheral IFNγ occurred after treatment with NIVO + RELA versus NIVO alone. A longer PFS was observed in patients treated with NIVO + RELA whose tumors had low CD8 expression compared with the NIVO arm. When evaluating the co-expression of CD8 and LAG-3, patients whose tumors had high CD8+LAG-3+ also showed a PFS benefit with NIVO + RELA versus NIVO. RNA sequencing revealed several distinct gene signatures associated with response to NIVO + RELA.
Conclusions: These results highlight the unique biological effects of RELA in combination with NIVO and provide further understanding of the patient characteristics associated with increased benefit from NIVO + RELA.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
E.J. Lipson reports grants from Bristol Myers Squibb during the conduct of the study; grants and personal fees from Bristol Myers Squibb, Merck, Regeneron Pharmaceuticals, and Sanofi; and personal fees from CareDx, Genentech, Instil Bio, Natera, Nektar Therapeutics, Pfizer, Replimune, Sun Pharma, IO Biotech, HUYA Bioscience, Novartis, OncoSec, and Merck outside the submitted work; and stock ownership (<$10K) in Iovance Biotherapeutics. S. Dolfi is an employee of Bristol Myers Squibb with stock benefits. H. Tang reports personal fees from Bristol Myers Squibb during the conduct of the study. H.A. Tawbi reports personal fees from Bristol Myers Squibb, Corcept Therapeutics, IO Biotech, Immunocore Holdings, Iovance Biotherapeutics, Krystal Biotech, Medicenna, Pfizer, Strand Therapeutics, and T-knife Therapeutics; grants and personal fees from Eisai, Merck, Novartis, and Regeneron Pharmaceuticals; and grants from Dragonfly Therapeutics, Genentech, GSK, and Syntrix Pharmaceuticals outside the submitted work. P. Rutkowski reports personal fees from MSD, Bristol Myers Squibb, Pierre Fabre, Medison Pharma, GENESIS Pharma, and Novartis outside the submitted work. H. Gogas reports grants from Bristol Myers Squibb during the conduct of the study; personal fees from MSD, Regeneron Pharmaceuticals, Pierre Fabre, and Pfizer; and grants from GENESIS Pharma, Pfizer, and Pierre Fabre outside the submitted work. P.A. Ascierto reports grants and personal fees from Bristol Myers Squibb, Roche-Genentech, Regeneron Pharmaceuticals, and Pfizer; personal fees and other support from MSD, Pierre Fabre, BioAI Health, Replimune, and Philogen; and personal fees from Novartis, Sun Pharma, Immunocore Holdings, Italfarmaco, Boehringer Ingelheim, Nouscom, Medicenna, Valo Therapeutics, Bayer Healthcare Pharmaceuticals, Erasca, BioNTech, ANAVEON, Genmab, Menarini, and Incyte outside the submitted work. K. Desai reports other support from Bristol Myers Squibb outside the submitted work. M. Maio reports other support from Bristol Myers Squibb, Roche, MSD, Merck Serono, Eli Lilly and Company, Amgen, Novartis, GSK, SciClone Pharmaceuticals, iOnctura, Regeneron Pharmaceuticals, and Astex Pharmaceuticals during the conduct of the study, as well as other support from Bristol Myers Squibb, Roche, Merck Serono, MSD, Eli Lilly and Company, Amgen, Novartis, GSK, Regeneron Pharmaceuticals, Pierre Fabre, iOnctura, SciClone Pharmaceuticals, and Astex Pharmaceuticals outside the submitted work. A. Mazzei reports personal fees from Bristol Myers Squibb during the conduct of the study. S. Keidel reports other support from Bristol Myers Squibb during the conduct of the study, as well as other support from Moderna and AbbVie outside the submitted work. K. Miller-Moslin reports other support from Bristol Myers Squibb during the conduct of the study, as well as other support from Bristol Myers Squibb outside the submitted work. J.X. Yu reports employment with Bristol Myers Squibb and stock benefits as an employee. F.S. Hodi reports nonfinancial support and other support from Bristol Myers Squibb during the conduct of the study; grants and personal fees from Bristol Myers Squibb; and personal fees from Merck, Novartis, Compass Therapeutics, Apricity Health, Bicara Therapeutics, Checkpoint Therapeutics, BioEntre Therapeutics, Gossamer Bio, Iovance Biotherapeutics, CatalYm, Immunocore Holdings, Kairos Pharma, Rheos Medicines, Bayer Healthcare Pharmaceuticals, Zumutor Biologics, Corner Therapeutics, Puretech Health, Curis Lifesciences, AstraZeneca, Pliant Therapeutics, Solu Therapeutics, Vir Biotechnology, and 92Bio outside the submitted work; and a patent for Methods for Treating MICA-related Disorders (No. 20100111973) pending, a patent for Tumor Antigens and Uses Thereof (No. 7250291) issued, a patent for Angiopoietin-2 Biomarkers Predictive of Anti-immune Checkpoint Response (No, 20170248603) pending, a patent for Compositions and Methods for Identification, Assessment, Prevention, and Treatment of Melanoma using PD-L1 Isoforms (No, 20160340407) pending, a patent for Therapeutic Peptides (No, 20160046716) pending, a patent for Methods of Using Pembrolizumab and Trebananib pending, a patent for Vaccine Compositions and Methods for Restoring NKG2D Pathway Function against Cancers (No. 10279021) issued, a patent for Antibodies That Bind to MHC Class I Polypeptide-related Sequence A (No. 10106611) issued, a patent for Anti-galectin Antibody Biomarkers Predictive of Anti-immune Checkpoint and Anti-angiogenesis Responses (publication number: 20170343552) pending, and a patent for Antibodies against EDIL3 and Methods of Use Thereof pending. D. Schadendorf reports grants from Merck and personal fees from Merck, Bristol Myers Squibb, Pierre Fabre, AstraZeneca, Boehringer Ingelheim, Ipsen, Sun Pharma, Philogen, Replimune, BioAlta, Daiichi Sankyo, Formycon, BioNTech, Immatics, Immunocore Holdings, NeraCare, Merck Serono, Novartis, Regeneron Pharmaceuticals, Iovance Biotherapeutics, and SkylineDX outside the submitted work. G.V. Long reports personal fees from Agenus, Amgen, Array BioPharma, AstraZeneca, Bayer Healthcare Pharmaceuticals, BioNTech, Boehringer Ingelheim, Bristol Myers Squibb, Evaxion Biotech, Fortiva Biologics, GI Innovation, Hexal AG, Highlight Therapeutics, Immunocore Holdings, Innovent Biologics USA, IO Biotech, Iovance Biotherapeutics, MSD, Novartis, PHMR Limited, Pierre Fabre, Regeneron Pharmaceuticals, Scancell, and SkylineDX outside the submitted work. C. Garnett-Benson is an employee of Bristol Myers Squibb with stock benefits. No disclosures were reported by the other authors.
Figures





References
-
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019;381:1535–46. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

