Sphingolipid synthesis maintains nuclear membrane integrity and genome stability during cell division
- PMID: 40608049
- PMCID: PMC12237252
- DOI: 10.1083/jcb.202407209
Sphingolipid synthesis maintains nuclear membrane integrity and genome stability during cell division
Abstract
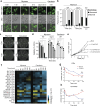
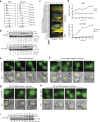
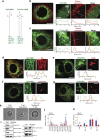
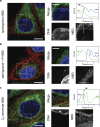
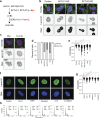
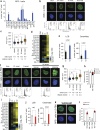
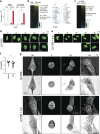
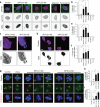
Lipid synthesis must be precisely regulated to support membrane growth and organelle biogenesis during cell division, yet little is known about how this process is coordinated with other cell cycle events. Here, we show that de novo synthesis of sphingolipids during the S and G2 phases of the cell cycle is essential to increasing nuclear membranes. Indeed, the products of serine palmitoyltransferase (SPT), long-chain bases, localize to the nucleus and are integral components of nuclear membranes in yeast and human cells. Importantly, inhibition of SPT fails to induce cell cycle arrest, causing nuclear membrane collapse and loss of viability in yeast cells. In human cells, this causes abnormal nuclear morphology and genomic instability, evidenced by the increased incidence of nuclear blebs, micronuclei, anaphase bridges, and multipolar mitosis. These results indicate that dysregulated cell division under low sphingolipid availability can drive several disease-associated phenotypes, including aberrant nuclear morphologies and genomic instability.
© 2025 Hwang et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures







References
MeSH terms
Substances
Grants and funding
- 26738-2021/Italian Association for Cancer Research
- Fondazione Cariplo
- Ministero dell'Istruzione, dell'Università e della Ricerca
- GR-2018-12367077/Ricerca Finalizzata
- 31023/Italian Association for Cancer Research
- 21665/Italian Association for Cancer Research
- Fondazione IEO-Monzino
- Italian Ministry of Health with Ricerca Corrente
- 1R01HD107873-01A1/NH/NIH HHS/United States
- R01 HD107873/HD/NICHD NIH HHS/United States
- 29228/Italian Association for Cancer Research
- 31556-2024/Italian Association for Cancer Research
LinkOut - more resources
Full Text Sources

