Melatonin for prevention of delirium in patients receiving mechanical ventilation in the intensive care unit: a multiarm multistage adaptive randomized controlled clinical trial (DEMEL)
- PMID: 40608082
- PMCID: PMC12283818
- DOI: 10.1007/s00134-025-08002-z
Melatonin for prevention of delirium in patients receiving mechanical ventilation in the intensive care unit: a multiarm multistage adaptive randomized controlled clinical trial (DEMEL)
Abstract
Purpose: To determine the dose of melatonin with an optimal pharmacokinetic profile and to test whether this dose reduces the prevalence of delirium in mechanically ventilated ICU patients as compared to placebo.
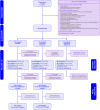
Methods: DEMEL, a multicenter adaptive phase 2b/3 randomized, placebo-controlled, double-blind trial included patients at 20 health centers in France from February 1st, 2019 through January 5th, 2021. Patients were randomized (1:1:1) to receive either placebo or low (0.3 mg) or high (3 mg) dose melatonin enterally at 9:00 p.m. for 14 consecutive nights or until death or ICU discharge, whichever came first. The interim primary endpoint (activity stage) was the percentage of patients who achieved an optimal melatonin pharmacokinetic profile 24 h after starting study treatment; the final primary endpoint (efficacy phase) was the percentage of patients who experienced delirium between randomization and day 14 (or until death or ICU discharge, whichever came first). Delirium was assessed twice daily using the Confusion Assessment Method for ICU.
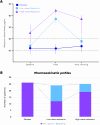
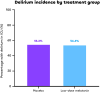
Results: We randomized 355 patients and included 334 in the primary analysis. At the preplanned analysis of the activity stage performed in 75 patients, the low-dose melatonin group had the highest rate of optimal pharmacokinetic profiles (12/24, 50%) when compared with the high-dose melatonin group (6/25, 24%) and the placebo group (0/26). Therefore, the Steering Committee recommended that the high-dose melatonin group be discontinued and that the low-dose melatonin group be selected to continue in the efficacy phase along with the placebo group. At the end of the efficacy stage, there was no difference in the final primary outcome of delirium incidence between the low-dose melatonin group and the placebo group: 80/147 (54.4%) vs 85/154 (55.2%), risk ratio, 0.986 [95% CI 0.803 to 1.211]; key secondary outcomes were also similar between groups. These included sleep quality, delirium-free, coma-free, and ventilator-free days at day 28; ICU and hospital length of stay; mortality at day 28, in the ICU, and in hospital; as well as long-term outcomes such as quality of life and postintensive care syndrome at day 90.
Conclusions: This randomized clinical trial found that the low-dose of melatonin (0.3 mg nightly) achieved a better pharmacokinetic profile than the high-dose (3 mg nightly), but did not change the incidence of delirium compared to placebo in mechanically ventilated critically-ill patients.
Trial registration: ClinicalTrial.gov website (NCT03524937).
Keywords: Delirium; Melatonin; Pharmacokinetics; Ventilation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: AMD reports grants from and personal fees from Fischer Paykel, Baxter, Air Liquide, and Addmedica, all outside the submitted work. KR received lecture fees from MSD, Shionogi, and a travel grant from Pfizer outside the submitted work. JDR reports grants and personal fees from Fischer Paykel. NT disclosed payment or honoraria for lectures, presentations from Fisher&Paykel outside this work, NT is supported by Pfizer and Gilead for attending meetings and/or travel outside this work. SN reports personal fees from Pfizer, Shionogi, Biomérieux, Fisher and Peykel, Mundi Pharma and Medtronic. RS reports a grant from LFB outside the submitted work. No other disclosures were reported.
Figures
References
-
- Olofsson K, Alling C, Lundberg D, Malmros C (2004) Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand 48:679–684. 10.1111/j.0001-5172.2004.00401.x - PubMed
-
- Dessap AM, Roche-Campo F, Launay J-M et al (2015) Delirium and circadian rhythm of melatonin during weaning from mechanical ventilation: an ancillary study of a weaning trial. Chest 148:1231–1241. 10.1378/chest.15-0525 - PubMed
-
- Claustrat B, Leston J (2015) Melatonin: physiological effects in humans. Neurochirurgie 61:77–84. 10.1016/j.neuchi.2015.03.002 - PubMed
-
- Lewy AJ, Wehr TA, Goodwin FK et al (1980) Light suppresses melatonin secretion in humans. Science 210:1267–1269. 10.1126/science.7434030 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

