Rare and abundant taxa in Artemisia desertorum rhizosphere soils demonstrate disparate responses to drought stress
- PMID: 40608163
- PMCID: PMC12229302
- DOI: 10.1007/s44307-025-00070-y
Rare and abundant taxa in Artemisia desertorum rhizosphere soils demonstrate disparate responses to drought stress
Abstract
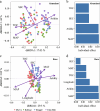
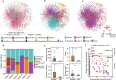
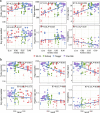
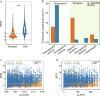
The growth and adaptability of desert plants depend on their rhizosphere microbes, which consist of a few abundant taxa and numerically dominant rare taxa. However, the differences in diversity, community structure, and functions of abundant and rare taxa in the rhizosphere microbiome of the same plant in different environments remain unclear. This study focuses on the rhizosphere microbial communities of Artemisia desertorum, a quintessential desert sand-stabilizing plant, investigating the diversity patterns and assembly processes of rare and abundant taxa across four Chinese deserts: Mu Us, Kubuqi, Tengger, and Ulan Buh. The results show that climatic factors, especially aridity and mean annual precipitation (MAP), significantly influence bacterial community composition and microbial network complexity. The interactions between rare and non-rare taxa are non-random, forming a modular network in which rare taxa serve as central nodes, and their loss could destabilize the network. Rare taxa are primarily shaped by heterogeneous selection, whereas abundant taxa are mainly influenced by dispersal limitation. Functionally, abundant taxa exhibit higher metabolic potential, whereas rare taxa are more involved in processes such as cell motility, indicating distinct ecological roles. These results provide new insights into the ecological functions of rare and abundant taxa in desert rhizosphere communities and highlight the importance of microbial management for desert plant health.
Keywords: Artemisia desertorum; Community assembly process; Desert area; Network complexity; Rhizosphere bacteria.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures


Similar articles
-
Drought-driven shifts in Eucommia ulmoides rhizosphere mycobiota and metabolites mediate host tolerance.Microbiol Spectr. 2025 Aug 5;13(8):e0084725. doi: 10.1128/spectrum.00847-25. Epub 2025 Jul 11. Microbiol Spectr. 2025. PMID: 40642984 Free PMC article.
-
Responses of rhizosphere bacterial communities with different niche breadths to liquid fertilizer produced from Fuji apple wastes during planting process.Microbiol Spectr. 2025 Jul;13(7):e0206824. doi: 10.1128/spectrum.02068-24. Epub 2025 May 30. Microbiol Spectr. 2025. PMID: 40445252 Free PMC article.
-
Effects of saffron-grape intercropping on saffron flower number and rhizosphere microbial community.BMC Microbiol. 2024 Dec 30;24(1):551. doi: 10.1186/s12866-024-03716-4. BMC Microbiol. 2024. PMID: 39736513 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
References
-
- Ala M, Jiang D, Niu C. The applicable density of sand-fixing shrub plantation in Horqin Sand Land of Northeastern China. Ecol Eng. 2014;64:250–4. 10.1016/j.ecoleng.2013.12.026.
-
- Banerjee S, Schlaeppi K, Heijden MGAVD. Keystone taxa as drivers of microbiome structure and functioning. Nat Rev Microbiol. 2018;16:567–76. 10.1038/s41579-018-0024-1. - PubMed
-
- Blaya J, López-Mondéjar R, Lloret E, Pascual JA, Ros M. Changes induced by Trichoderma harzianum in suppressive compost controlling Fusarium wilt. Pestic Biochem Physiol. 2013;107:112–9. 10.1016/j.pestbp.2013.06.001. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
