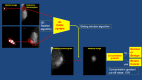
Development of a deep learning-based automated diagnostic system (DLADS) for classifying mammographic lesions - a first large-scale multi-institutional clinical trial in Japan
- PMID: 40608200
- PMCID: PMC12394324
- DOI: 10.1007/s12282-025-01741-3
Development of a deep learning-based automated diagnostic system (DLADS) for classifying mammographic lesions - a first large-scale multi-institutional clinical trial in Japan
Abstract
Background: Recently, western countries have built evidence on mammographic artificial Intelligence-computer-aided diagnosis (AI-CADx) systems; however, their effectiveness has not yet been sufficiently validated in Japanese women. In this study, we aimed to establish a Japanese mammographic AI-CADx system for the first time.
Methods: We retrospectively collected screening or diagnostic mammograms from 63 institutions in Japan. We then randomly divided the images into training, validation, and test datasets in a balanced ratio of 8:1:1 on a case-level basis. The gold standard of annotation for the AI-CADx system is mammographic findings based on pathologic references. The AI-CADx system was developed using SE-ResNet modules and a sliding window algorithm. A cut-off concentration gradient of the heatmap image was set at 15%. The AI-CADx system was considered accurate if it detected the presence of a malignant lesion in a breast cancer mammogram. The primary endpoint of the AI-CADx system was defined as a sensitivity and specificity of over 80% for breast cancer diagnosis in the test dataset.
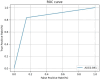
Results: We collected 20,638 mammograms from 11,450 Japanese women with a median age of 55 years. The mammograms included 5019 breast cancer (24.3%), 5026 benign (24.4%), and 10,593 normal (51.3%) mammograms. In the test dataset of 2059 mammograms, the AI-CADx system achieved a sensitivity of 83.5% and a specificity of 84.7% for breast cancer diagnosis. The AUC in the test dataset was 0.841 (DeLong 95% CI; 0.822-0.859). The Accuracy was almost consistent independent of breast density, mammographic findings, type of cancer, and mammography vendors (AUC (range); 0.639-0.906).
Conclusions: The developed Japanese mammographic AI-CADx system diagnosed breast cancer with a pre-specified sensitivity and specificity. We are planning a prospective study to validate the breast cancer diagnostic performance of Japanese physicians using this AI-CADx system as a second reader.
Trial registration: UMIN, trial number UMIN000039009. Registered 26 December 2019, https://www.umin.ac.jp/ctr/.
Keywords: Artificial intelligence; Breast cancer; Computer-aided detection; Computer-aided diagnosis; Convolutional neural network; Mammography.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: All authors declared no conflicts of interest. Ethical approval and consent to participate: This trial was approved by the institutional review board of National Cancer Center Hospital East (Approval number: 2019–048). The need for signed informed consent was waived because of a retrospective study without patient interactions.
Figures
References
-
- Independent UKPoBCS. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380:1778–86. - PubMed
-
- Taylor P, Potts HW. Computer aids and human second reading as interventions in screening mammography: two systematic reviews to compare effects on cancer detection and recall rate. Eur J Cancer. 2008;44:798–807. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical