Functional and Anatomical Outcomes of Faricimab in Previously Treated Wet Age-Related Macular Degeneration: Systematic Review and Pooled Analysis
- PMID: 40608264
- PMCID: PMC12271014
- DOI: 10.1007/s40123-025-01181-4
Functional and Anatomical Outcomes of Faricimab in Previously Treated Wet Age-Related Macular Degeneration: Systematic Review and Pooled Analysis
Erratum in
-
Correction: Functional and Anatomical Outcomes of Faricimab in Previously Treated Wet Age-Related Macular Degeneration: Systematic Review and Pooled Analysis.Ophthalmol Ther. 2025 Oct;14(10):2621. doi: 10.1007/s40123-025-01224-w. Ophthalmol Ther. 2025. PMID: 40802164 Free PMC article. No abstract available.
Abstract
Introduction: To evaluate the outcomes of intravitreal faricimab (IVF; Vabysmo®) in previously treated patients with wet age-related macular degeneration (wAMD), focusing on best available visual acuity (BAVA), central subfield thickness (CST), injection interval, complications, fluid resolution, and reversion rates to prior therapies.
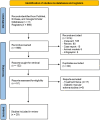
Methods: The PubMed, Embase, and Google Scholar databases were searched for studies reporting outcomes of treatments for previously treated cases of wAMD. Mean differences (MD) with 95% confidence intervals (CI) were used to compute the effect size of the change in outcomes.
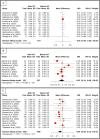
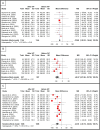
Results: A total of 29 studies with 2070 patients (1003 women, mean age 78.9 years) and 2128 eyes were included. BAVA and CST were reported in 28 studies, fluid status in 21, injection interval in 14, and reversion rates in 6. Pooled analysis showed significant but modest improvement in BAVA when IVF was given for > 6 months (MD = -0.026 LogMAR, p < 0.05) but not at earlier follow-ups. A similar trend was noted with injection interval extension when IVF was given beyond 6 months (MD = +2.1 weeks, p < 0.05). CST reduction was observed at all time points (overall MD = -37.7 μm, p < 0.05). Complication rates were reported in nine studies, with an overall rate of 1.2%, including retinal pigment epithelium tear, intraocular inflammation, endophthalmitis, and subretinal hemorrhage. Reversion to prior or other anti-vascular endothelial growth factor (anti-VEGF) therapy was reported in six studies, occurring in 23% of eyes.
Conclusions: We reported the outcomes of utilizing IVF in previously treated cases of wAMD. IVF showed a significant improvement in CST at all time points and in the extension of injection interval. Visual outcomes remained unchanged when followed for less than 6 months but improved significantly but modestly when followed for more than 6 months. Switching to intravitreal faricimab may be a useful treatment option for previously treated patients with wAMD, with a goal of reducing treatment burden and improving treatment efficacy.
Keywords: AMD; Age-related macular degeneration; Anti-VEGF; Faricimab; Intravitreal therapy; Retina; Treatment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: Chirag Shah is a consultant and clinical trialist for Genentech. Andre Witkin is a consultant and primary investigator for Genentech and Apellis and an Editorial Board member of Ophthalmology and Therapy. Andre Witkin was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Jeffrey Heier is a consultant for: 4DMT, Abbvie/Regenxbio, Abpro, Affamed, Alkeus, Annexon, Asclepix, Aviceda, Bayer, Beacon, Boehringer Ingleheim, Breye Therapeutics, Caeregen, Cogent, Complement Therapeutics, Curacle, Daiichi Sankyo, Emmetrope, Endogena, Frontera, Galimedix, Inflammx, Kaigene, Kanghong, Lilly, Manistee, Nanoscope, Notal Vision, Novartis, Ocuphire, OcuTerra, Opthea, Osanni, Ray Therapeutics, Regeneron, Samsung Bioepis, Sanofi, Skyline, Stealth, Laboratoires Thea, Unity Bio, Vanotech, and Visgenx; member of DSMC: Akouos; employee/executive: Chief Scientific Officer: Ocular Therapeutix; research investigator: 4DMT, Abbvie/Regenxbio, Annexon, Apellis, Astellas, Bayer, Beacon, Boehringer Ingleheim, Cognition Therapeutics, Curacle, Genentech/Roche, Janssen R&D, Kodiak, Notal Vision, Novartis, Oculis, Opthea, Perceive Bio, Regeneron, Sanofi, Skyline, Stealth Biotherapeutics, and Vanotech; holds equity in: Adverum, Aldeyra, Alzheon, Aviceda, Caeregen, Inflammx, jCyte, Manistee, Ocuphire, Ocular Therapeutix, Osanni Bio, Ray Therapeutics, RevOpsis, Vinci, Visgenx, and Vitranu. Ali Khodor, Jonathan T. Caranfa, Tavish Nanda, Raul E. Ruiz-Lozano, Manuel E. Quiroga-Garza, Stephanie Choi, Ali Chehab, and Eugenia M. Ramos-Dávila declare no conflicts of interest. Ethical Approval: This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Figures

References
-
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–16. - PubMed
-
- Maguire MG, Martin DF, Ying GS, Jaffe GJ, Daniel E, Grunwald JE, et al. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123(8):1751–61. - PMC - PubMed
-
- Rubio RG, Adamis AP. Ocular angiogenesis: vascular endothelial growth factor and other factors. Dev Ophthalmol. 2016;55:28–37. - PubMed
LinkOut - more resources
Full Text Sources

