Protocol for monitoring of neutralizing antibodies to various receptor-binding domains using the tANCHOR system
- PMID: 40608511
- PMCID: PMC12270789
- DOI: 10.1016/j.xpro.2025.103926
Protocol for monitoring of neutralizing antibodies to various receptor-binding domains using the tANCHOR system
Abstract
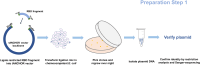
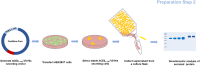
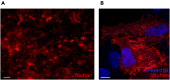
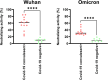
Neutralizing antibody analysis against SARS-CoV-2 variants requires assays that rapidly adapt to protein mutations. Here, we present a protocol for monitoring neutralizing antibodies to various receptor-binding domains using the tANCHOR system. We describe steps for displaying variant receptor-binding domains on HeLa cells and producing tagged soluble angiotensin-converting enzyme 2 (ACE2). We then detail the procedures for establishing a cell-based ELISA to measure serum neutralization efficiency, using ACE2 competition as a readout. For complete details on the use and execution of this protocol, please refer to Ivanusic et al.1.
Keywords: Antibody; Cell Membrane; Cell-based Assays; High-Throughput Screening; Immunology; Microbiology; Protein expression and purification.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The non-profit organization Peter und Traudl Engelhorn Foundation holds a patent application for the tANCHOR system, where D.I. is listed as an inventor.
Figures





Similar articles
-
A New Measure of Quantified Social Health Is Associated With Levels of Discomfort, Capability, and Mental and General Health Among Patients Seeking Musculoskeletal Specialty Care.Clin Orthop Relat Res. 2025 Apr 1;483(4):647-663. doi: 10.1097/CORR.0000000000003394. Epub 2025 Feb 5. Clin Orthop Relat Res. 2025. PMID: 39915110
-
Antibody Levels From High-Throughput Variant-Specific SARS-CoV-2 Anti-Spike Immunoglobulin G and Angiotensin-Converting Enzyme 2 Neutralization Assays Correlate With COVID-19 Infection Risk in a Large Population.J Infect Dis. 2025 Apr 15;231(4):921-930. doi: 10.1093/infdis/jiae622. J Infect Dis. 2025. PMID: 39676529
-
Determinants of susceptibility to SARS-CoV-2 infection in murine ACE2.J Virol. 2025 Jun 17;99(6):e0054325. doi: 10.1128/jvi.00543-25. Epub 2025 May 12. J Virol. 2025. PMID: 40353671 Free PMC article.
-
Laboratory-based molecular test alternatives to RT-PCR for the diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Oct 14;10(10):CD015618. doi: 10.1002/14651858.CD015618. Cochrane Database Syst Rev. 2024. PMID: 39400904
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
References
-
- Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., COVID-19 Genomics UK COG-UK Consortium, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

