Prolonged anoxic exposure impacts antibiotic sensitivity profiles of Pseudomonas aeruginosa
- PMID: 40608522
- PMCID: PMC12254951
- DOI: 10.1093/femsle/fnaf066
Prolonged anoxic exposure impacts antibiotic sensitivity profiles of Pseudomonas aeruginosa
Abstract
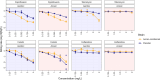
Chronic respiratory tract infections with Pseudomonas aeruginosa frequently occur in patients with cystic fibrosis, chronic obstructive pulmonary disease, and bronchiectasis. A hallmark of these conditions is the accumulation of mucus plugs, creating oxygen-limited niches. Within these microenvironments, P. aeruginosa undergoes cellular modifications that may alter its antibiotic sensitivity. Although the acute effects of anoxia are well studied, the impact of prolonged anoxic exposure on antibiotic sensitivity remains unclear. In this study, we developed anoxic-conditioned P. aeruginosa strains by passaging a laboratory strain for 22 days in an anoxic environment. We performed time-kill assays with both parental and anoxic-conditioned strains in anoxic and aerobic environments, using ceftazidime, ciprofloxacin, colistin, and tobramycin. The anoxic-conditioned strains exhibited increased susceptibility to tobramycin and reduced sensitivity to colistin and ceftazidime. These differences were attributed to altered killing rates (as with tobramycin) or reduced regrowth under anoxic conditions (as with colistin). For ciprofloxacin, a steeper killing rate was observed against the anoxic-conditioned strains, but 24-h outcomes were similar to the parental strain. Overall, our findings demonstrate that long-term anoxia alters antibiotic sensitivity in P. aeruginosa differently than acute anoxia, with important implications for treating chronic infections in oxygen-limited environments.
Keywords: Pseudomonas aeruginosa; anoxia; antibiotic sensitivity; metabolic specialization; oxygen.
© The Author(s) 2025. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
None declared.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

