Galectin-3 inhibition ameliorates hepatic steatosis in a multilineage 3D spheroid model
- PMID: 40608687
- PMCID: PMC12225867
- DOI: 10.1371/journal.pone.0326373
Galectin-3 inhibition ameliorates hepatic steatosis in a multilineage 3D spheroid model
Abstract
Background: Metabolic dysfunction-associated steatotic liver disease (MASLD) is the leading cause of chronic liver disease, and liver-related morbidity and mortality worldwide. MASLD is a multifactorial condition, which still needs to be completely understood. Galectin 3 (Gal-3) is up-regulated in several liver disorders suggesting its implication in the mechanisms underlying liver damage.
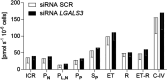
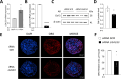
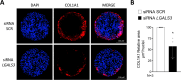
Methods: A human multilineage 3D model was utilized to investigate the role of Gal-3 in MASLD development. Human hepatoma cell line (HepG2) and human stellate cell line (LX-2) were co-cultured in a physiological ratio of 24:1 and treated with a mixture of palmitic and oleic acid (PAOA, ratio 1:2) to induce hepatocyte steatosis and facilitate the development of fibrosis. While the effect of LGALS3 silencing on neutral fat content was assessed by Oil-Red-O (ORO) staining, type I collagen production was analysed by immunofluorescent staining for collagen type I alpha 1 (COL1A1).
Results: Gal-3 depletion caused a reduction of neutral lipid content and COL1A1 accumulation in 3D spheroids. While LGALS3 silencing did not significantly alter the respiratory state, analysis of genes involved in lipid metabolism demonstrated significant changes in genes involved in β-oxidation and triglyceride synthesis.
Conclusion: These results suggest a role of Gal-3 in the regulation of fatty acid and collagen accumulation, thereby indicating that approaches aimed at inhibiting Gal-3 may represent a promising therapeutic strategy in MASLD.
Copyright: © 2025 Sedda et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

