The non-structural protein of SFTSV activates NLRP1 and CARD8 inflammasome through disrupting the DPP9-mediated ternary complex
- PMID: 40608794
- PMCID: PMC12225885
- DOI: 10.1371/journal.ppat.1013258
The non-structural protein of SFTSV activates NLRP1 and CARD8 inflammasome through disrupting the DPP9-mediated ternary complex
Abstract
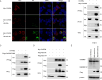
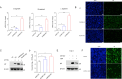
Inflammasomes function as immune-signaling platforms that were assembled following detection of pathogens. NLRP1 and CARD8 are related inflammasomes that use their C-terminal (CT) fragments containing a caspase recruitment domain (CARD) and the UPA domain to initiate the inflammasome. At rest, dipeptidyl peptidases 8 and 9 (DPP8/9) inhibit inflammatory CT by interacting with the function-to-find domain (FIIND) of NLRP1/CARD8 and forming an inhibitory NLRP1/CARD8-DPP9 ternary complex consisting of DPP9, full-length NLRP1/CARD8, and NLRP1/CARD8 CT. However, the specific triggers of NLRP1 and CARD8 have not yet been fully identified. Here, we report that a tick-borne bunyavirus SFTSV infection activates the NLRP1 inflammasome in primary keratinocytes and the CARD8 inflammasome in macrophages in a similar manner by targeting the ternary inhibitory complex, respectively. Mechanistically, SFTSV NSs interact with NLRP1 and CARD8 via their FIIND domains, suggesting that DPP8/9 are likely to compete for binding; on the other hand, NSs promote the degradation of DPP8 and DPP9. Both contribute to more efficient destabilization of the DPP8/9 ternary complex and release the activated CT. Moreover, CARD8 deletion promotes SFTSV replication. In conclusion, we found a novel mechanism of viral protein activation of NLRP1 and CARD8 by disrupting the DPP9-binding checkpoint.
Copyright: © 2025 Liu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Human DPP9 represses NLRP1 inflammasome and protects against autoinflammatory diseases via both peptidase activity and FIIND domain binding.J Biol Chem. 2018 Dec 7;293(49):18864-18878. doi: 10.1074/jbc.RA118.004350. Epub 2018 Oct 5. J Biol Chem. 2018. PMID: 30291141 Free PMC article.
-
Diverse autoinhibitory mechanisms of FIIND-containing proteins: Insight into regulation of NLRP1 and CARD8 inflammasome.PLoS Pathog. 2025 Jan 24;21(1):e1012877. doi: 10.1371/journal.ppat.1012877. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39854601 Free PMC article.
-
DPP9's Enzymatic Activity and Not Its Binding to CARD8 Inhibits Inflammasome Activation.ACS Chem Biol. 2019 Nov 15;14(11):2424-2429. doi: 10.1021/acschembio.9b00462. Epub 2019 Sep 20. ACS Chem Biol. 2019. PMID: 31525884 Free PMC article.
-
Mechanistic insights from inflammasome structures.Nat Rev Immunol. 2024 Jul;24(7):518-535. doi: 10.1038/s41577-024-00995-w. Epub 2024 Feb 19. Nat Rev Immunol. 2024. PMID: 38374299 Free PMC article. Review.
-
The NLRP1 Inflammasome in Human Skin and Beyond.Int J Mol Sci. 2020 Jul 6;21(13):4788. doi: 10.3390/ijms21134788. Int J Mol Sci. 2020. PMID: 32640751 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

