Semaglutide ameliorates diabetes-associated cognitive dysfunction in mouse model of type 2 diabetes
- PMID: 40608828
- PMCID: PMC12225814
- DOI: 10.1371/journal.pone.0326897
Semaglutide ameliorates diabetes-associated cognitive dysfunction in mouse model of type 2 diabetes
Abstract
Background: Type 2 diabetes mellitus (T2DM) is associated with cognitive dysfunction, which significantly impacts the quality of life. Semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, has shown potential neuroprotective effects. This study investigates the efficacy of semaglutide in ameliorating cognitive dysfunction in a mouse model of T2DM.
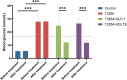
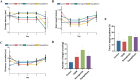
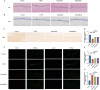
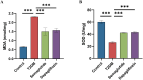
Methods: Male C57BL/6J mice were fed a high-fat diet for four weeks and received a single intraperitoneal injection of streptozotocin (150 mg/kg) to induce T2DM. All mice were divided into four groups: control, diabetes control (T2DM), semaglutide treatment (semaglutide, 0.1 mg/kg) and dapagliflozin treatment (dapagliflozin 1 mg/kg). Cognitive function was assessed using the Morris water maze (MWM) test. Histomorphological analysis of hippocampal tissues was performed using H&E and Nissl staining. Immunofluorescence was used to assess LRP1 expression and apoptosis. Biochemical analyses measured oxidative stress markers (SOD, MDA) and inflammatory cytokines (IL-1β, IL-6, TNF-α, CRP).
Results: Semaglutide treatment significantly reduced blood glucose levels in diabetic mice. In the MWM test, semaglutide-treated mice showed reduced escape latencies, indicating improved spatial learning and memory. Histomorphological analysis revealed preserved neuronal structure in the hippocampus with reduced neuronal damage and apoptosis in the semaglutide-treated group. Immunofluorescence showed increased LRP1 expression and decreased apoptosis. Biochemical analyses indicated that semaglutide reduced oxidative stress and inflammatory markers, further supporting its neuroprotective effects.
Conclusions: Semaglutide effectively ameliorates cognitive dysfunction in T2DM mice, likely through mechanisms involving the reduction of oxidative stress, inflammation, and neuronal apoptosis. These findings suggest that semaglutide has potential as a therapeutic agent for managing diabetes-associated cognitive decline. Further research, including long-term studies and clinical trials, is necessary to validate these findings and explore the broader applicability of semaglutide in treating cognitive impairments in diabetic patients.
Copyright: © 2025 Zhu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Human placental extract rescues hippocampal damage associated with cognitive impairment in diabetic male rats through antioxidative, anti-inflammatory, and neuromodulatory activities.Metab Brain Dis. 2025 Jun 16;40(6):225. doi: 10.1007/s11011-025-01646-2. Metab Brain Dis. 2025. PMID: 40522516 Free PMC article.
-
Mazdutide, a dual agonist targeting GLP-1R and GCGR, mitigates diabetes-associated cognitive dysfunction: mechanistic insights from multi-omics analysis.EBioMedicine. 2025 Jul;117:105791. doi: 10.1016/j.ebiom.2025.105791. Epub 2025 Jun 6. EBioMedicine. 2025. PMID: 40479843 Free PMC article.
-
Tirzepatide mitigates cognitive decline in zebrafish model of type 2 diabetes mellitus induced by high-fat diet.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jul;398(7):8861-8883. doi: 10.1007/s00210-025-03827-3. Epub 2025 Jan 28. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39873719
-
Dipeptidyl-peptidase (DPP)-4 inhibitors and glucagon-like peptide (GLP)-1 analogues for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk for the development of type 2 diabetes mellitus.Cochrane Database Syst Rev. 2017 May 10;5(5):CD012204. doi: 10.1002/14651858.CD012204.pub2. Cochrane Database Syst Rev. 2017. PMID: 28489279 Free PMC article.
-
A Systematic Review of Semaglutide's Influence on Cognitive Function in Preclinical Animal Models and Cell-Line Studies.Int J Mol Sci. 2024 May 2;25(9):4972. doi: 10.3390/ijms25094972. Int J Mol Sci. 2024. PMID: 38732190 Free PMC article.
References
-
- Su J, Sun G, An J, Ao Y, Li J, Shen Z, et al. Efficacy and safety of the integration of traditional Chinese medicine and western medicine in the treatment of diabetes-associated cognitive decline: a systematic review and meta-analysis. Front Pharmacol. 2023;14:1280736. doi: 10.3389/fphar.2023.1280736 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

