Chlorinated biscoumarins inhibit chikungunya virus replication in cell-based and animal models
- PMID: 40608982
- PMCID: PMC12305873
- DOI: 10.1080/22221751.2025.2529889
Chlorinated biscoumarins inhibit chikungunya virus replication in cell-based and animal models
Abstract
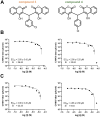
Biscoumarin derivatives were evaluated for antiviral activity against chikungunya virus (CHIKV), a re-emerging mosquito-borne alphavirus with no approved treatment. Compounds 3 and 4 demonstrated potent in vitro antiviral efficacy, with EC₅₀ values of 2.85 ± 0.42 µM and 3.08 ± 0.45 µM (SI > 20) for compound 3 in Vero and HEK293 cells, respectively. Compound 4 showed comparable potency in Vero cells but was less effective in HEK293 cells. Time-of-addition and replicon assays suggested that both compounds act at a post-entry step, likely inhibiting viral RNA replication. In vivo, a single oral dose of 250 mg/kg was well tolerated in mice and rats, with no signs of acute hepatorenal toxicity and favourable pharmacokinetic profiles. Compound 3 & 4 significantly reduced tissue viral loads within 24 hours; however, their antiviral effect diminished after the drug was cleared from circulation. Due to concerns about potential cumulative toxicity, repeated administration was avoided. Preliminary mechanistic studies indicated moderate inhibition of the viral nsP1 methyltransferase and suggested possible involvement of host pathways. These findings highlight biscoumarin derivatives - particularly compound 3 - as promising antiviral candidates against CHIKV, meriting further optimization and investigation into their mechanisms of action.
Keywords: Biscoumarin; chikungunya virus; efficacy; methyltransferase; mice; molecular docking.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures






References
-
- Roongaraya, P, Boonyasuppayakorn S.. Chikungunya vaccines: an update in 2023. Asian Pac J Allergy Immunol. 2023;41(1):1–11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
