Iron-catalyzed oxidative stress compromises cancer promotional effect of BRCA2 haploinsufficiency through mitochondria-targeted ferroptosis
- PMID: 40609478
- PMCID: PMC12270739
- DOI: 10.1016/j.redox.2025.103739
Iron-catalyzed oxidative stress compromises cancer promotional effect of BRCA2 haploinsufficiency through mitochondria-targeted ferroptosis
Abstract
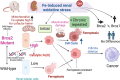
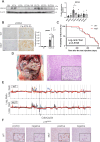
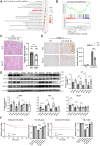
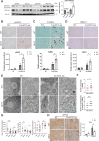
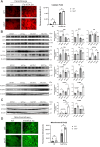
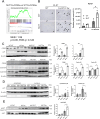
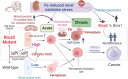
Pathogenic variants in BRCA2 are hereditary risks for various cancers, including breast, ovary, pancreas and prostate. Genomic instability due to insufficient homologous recombination is thought as responsible for carcinogenesis. Reportedly, endogenous or exogenous aldehydes, including formaldehyde and acetaldehyde, suppress BRCA2 function. However, molecular sequences how BRCA2 insufficiency leads to carcinogenesis remains unelucidated. To assess whether Fenton reaction-based oxidative stress is a promotional risk factor of carcinogenesis in BRCA2 haploinsufficiency, we here applied iron-induced renal carcinogenesis to a newly established rat heterozygous mutation model of Brca2 (mutant, T1942Kfs/+; MUT). Rat MUT model, despite significant increase in spontaneous malignant tumors, showed no promotional effect on renal carcinogenesis induced by ferric nitrilotriacetate (Fe-NTA) in contrast to our previous study using Brca1 mutant rats. Array-based comparative genome hybridization of renal cell carcinoma in MUT revealed significant increase in the frequency of homozygous Cdkn2A deletion. Whereas acute-phase analysis of the kidney after single or 1-week Fe-NTA administration to MUT showed suppressed lipid peroxidation, consistent with ferroptosis-resistance, ferroptosis and regeneration of tubular cells were coexistent with higher cytoplasmic catalytic Fe(II) levels in the subacute phase of MUT after 3-week Fe-NTA administration. Mechanistically, mitochondrial dysfunction with excess iron, promoted by insufficient BRCA2 presumably for maintaining DNA integrity, eventually initiated ferroptotic process. In conclusion, iron-dependent oxidative stress plays double-edged roles either for cell death or proliferation in carcinogenesis and its biological consequences are distinct between BRCA2 and BRCA1 haploinsufficiency. Our results suggest that iron-catalyzed oxidative stress is not a major driving force of carcinogenesis in BRCA2 pathogenic variants.
Keywords: BRCA2; Fe-NTA; Ferroptosis; Genome instability; Mitochondria.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
BRCA1 haploinsufficiency promotes chromosomal amplification under Fenton reaction-based carcinogenesis through ferroptosis-resistance.Redox Biol. 2022 Aug;54:102356. doi: 10.1016/j.redox.2022.102356. Epub 2022 May 28. Redox Biol. 2022. PMID: 35667247 Free PMC article.
-
Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations.Cochrane Database Syst Rev. 2018 Aug 24;8(8):CD012464. doi: 10.1002/14651858.CD012464.pub2. Cochrane Database Syst Rev. 2018. PMID: 30141832 Free PMC article.
-
M1 Macrophage-Derived TNF-α Promotes Pancreatic Cancer Ferroptosis Via p38 MAPK-ACSL4 Pathway.Curr Mol Med. 2025 Jul 10. doi: 10.2174/0115665240374551250630075409. Online ahead of print. Curr Mol Med. 2025. PMID: 40653839
-
Diagnostic test accuracy and cost-effectiveness of tests for codeletion of chromosomal arms 1p and 19q in people with glioma.Cochrane Database Syst Rev. 2022 Mar 2;3(3):CD013387. doi: 10.1002/14651858.CD013387.pub2. Cochrane Database Syst Rev. 2022. PMID: 35233774 Free PMC article.
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
References
-
- Wooster R., Bignell G., Lancaster J., Swift S., Seal S., Mangion J., Collins N., Gregory S., Gumbs C., Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378(6559):789–792. - PubMed
-
- Momozawa Y., Sasai R., Usui Y., Shiraishi K., Iwasaki Y., Taniyama Y., Parsons M.T., Mizukami K., Sekine Y., Hirata M., Kamatani Y., Endo M., Inai C., Takata S., Ito H., Kohno T., Matsuda K., Nakamura S., Sugano K., Yoshida T., Nakagawa H., Matsuo K., Murakami Y., Spurdle A.B., Kubo M. Expansion of cancer risk profile for BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2022;8(6):871–878. - PMC - PubMed
-
- Momozawa Y., Iwasaki Y., Parsons M.T., Kamatani Y., Takahashi A., Tamura C., Katagiri T., Yoshida T., Nakamura S., Sugano K., Miki Y., Hirata M., Matsuda K., Spurdle A.B., Kubo M. Germline pathogenic variants of 11 breast cancer genes in 7,051 Japanese patients and 11,241 controls. Nat. Commun. 2018;9(1):4083. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

