Erythropoietin decreases apoptosis and promotes Schwann cell repair and phagocytosis following nerve crush injury in mice
- PMID: 40610400
- PMCID: PMC12229349
- DOI: 10.1038/s41419-025-07825-4
Erythropoietin decreases apoptosis and promotes Schwann cell repair and phagocytosis following nerve crush injury in mice
Abstract
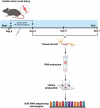
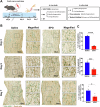
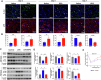
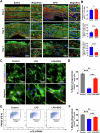
After peripheral nerve trauma, insufficient clearance of phagocytic debris significantly hinders nerve regeneration. Without sufficient myelin debris clearance, Schwann cells (SCs) undergo increased apoptosis, impairing functional recovery. There is no treatment for peripheral nerve crush injury (PNCI). Erythropoietin (EPO) is an FDA-approved drug for anemia, which may help in the treatment of PNCI by transdifferentiating resident SCs into repair SCs (rSCs) and enhancing phagocytosis to facilitate the removal of cellular debris. For the first time, we conducted bulk RNA sequencing on mice with calibrated sciatic nerve crush injuries (SNCIs) on days 3, 5, and 7 post-SNCI to uncover transcriptomic changes with and without EPO treatment. We found EPO altered several biological pathways and associated genes, particularly those involved in cell apoptosis, differentiation, proliferation, phagocytosis, myelination, and neurogenesis. We validated the effects of EPO on SNCI on early (day 3) and intermediate (days 5 and 7) post-SNCI, and found EPO treatment reduced apoptosis (TUNEL), and enhanced SC repair (c-Jun and p75), proliferation (Ki67), and the phagocytosis of myelin debris by rSCs at crush injury sites. This improvement corresponded with an enhanced sciatic functional index (SFI). We also confirmed these findings in-vitro. EPO significantly enhanced SC repair during early de-differentiation, marked by high c-Jun and p75 protein levels, and later re-differentiation with high EGR2 and low c-Jun and p75 levels. These changes occurred under lipopolysaccharide (LPS) stress at 24 and 72 h, respectively, compared to LPS treatment alone. Under LPS stress, EPO also significantly increased rSCs proliferation and phagocytosis of myelin or dead SCs. In conclusion, our findings support EPO may enhance the function of rSCs in debris clearance as a basis for its possible use in treating nerve trauma.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethical approval: Our manuscript does not contain any human data. Experimental design and animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at The University of Arizona College of Medicine, Tucson, AZ, and The Penn State University, Hershey, PA, USA. All experiments were conducted following the approved guidelines and regulations.
Figures



Update of
-
Erythropoietin decreases apoptosis and promotes Schwann cell repair and phagocytosis following nerve crush injury in mice.bioRxiv [Preprint]. 2025 Jan 23:2025.01.22.634402. doi: 10.1101/2025.01.22.634402. bioRxiv. 2025. Update in: Cell Death Dis. 2025 Jul 3;16(1):490. doi: 10.1038/s41419-025-07825-4. PMID: 39896684 Free PMC article. Updated. Preprint.
Similar articles
-
Erythropoietin decreases apoptosis and promotes Schwann cell repair and phagocytosis following nerve crush injury in mice.bioRxiv [Preprint]. 2025 Jan 23:2025.01.22.634402. doi: 10.1101/2025.01.22.634402. bioRxiv. 2025. Update in: Cell Death Dis. 2025 Jul 3;16(1):490. doi: 10.1038/s41419-025-07825-4. PMID: 39896684 Free PMC article. Updated. Preprint.
-
Etv5 Is Required for Peripheral Nerve Function and the Injury Response.eNeuro. 2025 Jul 23;12(7):ENEURO.0410-20.2025. doi: 10.1523/ENEURO.0410-20.2025. Print 2025 Jul. eNeuro. 2025. PMID: 40701803 Free PMC article.
-
Resveratrol regulates the recovery of rat sciatic nerve crush injury by promoting the autophagy of Schwann cells.Life Sci. 2020 Sep 1;256:117959. doi: 10.1016/j.lfs.2020.117959. Epub 2020 Jun 10. Life Sci. 2020. PMID: 32531375
-
A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment.Health Technol Assess. 2007 Apr;11(13):1-202, iii-iv. doi: 10.3310/hta11130. Health Technol Assess. 2007. PMID: 17408534
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
References
-
- Wang J-L, Huang Q-M, Hu D-X, Zhang W-J. Therapeutic effect of exosomes derived from Schwann cells in the repair of peripheral nerve injury. Life Sci. 2024;357:123086. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

