Comparable outcomes with 14-, 21-, or standard 28-day venetoclax in the first cycle of azacitidine-venetoclax in untreated acute myeloid leukemia: real-world experience from the Hokkaido Leukemia Net
- PMID: 40610408
- PMCID: PMC12229622
- DOI: 10.1038/s41408-025-01324-7
Comparable outcomes with 14-, 21-, or standard 28-day venetoclax in the first cycle of azacitidine-venetoclax in untreated acute myeloid leukemia: real-world experience from the Hokkaido Leukemia Net
Conflict of interest statement
Competing interests: MK's spouse is an employee of AbbVie GK. TK has received honoraria from Astellas Pharma, AbbVie, Amgen, Kyowa Kirin, Nippon Shinyaku, Ono Pharmaceutical, Otsuka Pharmaceutical, Novartis, Pfizer, and Bristol-Myers Squibb; and consultancy fees from Otsuka Pharmaceutical. TT has received honoraria from Abbvie, Astellas, AstraZeneca, Novartis, Bristol-Myers Squibb, Gilead, Nippon Shinyaku, Kyowa Kirin, Janssen, and research funding from LUCA Science, Priothera SAS, Pharma Essentia Japan, Abbvie, IQVIA, CSL Behring, Cmic, Gilead, Novartis, Janssen, Chugai, Kyowa Kirin, International Advanced Medical Organization, and subsidy or donation from Chugai, Kyowa Kirin, Sumitomo Pharma.
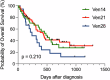
Figures
References
-
- Miyamoto T, Sanford D, Tomuleasa C, Hsiao HH, Olivera LJE, Enjeti AK, et al. Real-world treatment patterns and clinical outcomes in patients with AML unfit for first-line intensive chemotherapy. Leuk Lymphoma. 2022;63:928–38. - PubMed
-
- DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617–29. - PubMed
-
- Kanaya M, Mukai Y, Kobayashi M, Kajikawa S, Suzuki T, Yokoyama E, et al. Combination of low white blood cell count and impaired renal function is a risk factor for higher concentrations of venetoclax and prolonged neutropenia in AML patients with venetoclax combined regimen: real-world experience in the Japanese cohort. Blood. 2024;144:2930.
-
- Karrar O, Abdelmagid M, Rana M, Iftikhar M, McCullough K, Al-Kali A, et al. Venetoclax duration (14 vs. 21 vs. 28 days) in combination with hypomethylating agent in newly diagnosed acute myeloid leukemia: comparative analysis of response, toxicity, and survival. Am J Hematol. 2024;99:E63–E66. - PubMed
Publication types
LinkOut - more resources
Full Text Sources