FL3 mitigates cardiac ischemia-reperfusion injury by promoting mitochondrial fusion to restore calcium homeostasis
- PMID: 40610427
- PMCID: PMC12229567
- DOI: 10.1038/s41420-025-02575-w
FL3 mitigates cardiac ischemia-reperfusion injury by promoting mitochondrial fusion to restore calcium homeostasis
Abstract
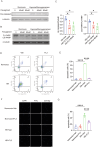
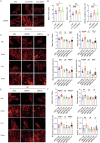
This study aims to investigate the therapeutic potential of Flavagline3 (FL3) in mitigating myocardial ischemia-reperfusion (IR) injury, with a specific focus on its regulatory effects on mitochondrial fusion, mitochondrial-endoplasmic reticulum (ER) interactions, and calcium homeostasis in cardiomyocytes. Using a well-established myocardial IR injury model in mice and primary cardiomyocytes treated with FL3, the study assessed its impact on mitochondrial dynamics and intracellular signaling processes. The results demonstrated that FL3 effectively reduced myocardial apoptosis, infarct size, and cardiac dysfunction caused by IR injury. Mechanistically, FL3 promoted mitochondrial fusion in a mitofusin1 (MFN1)-dependent manner, preserving mitochondrial function under stress conditions and enhancing cellular resilience. Furthermore, FL3 facilitated mitochondrial-ER crosstalk, which played a critical role in modulating intracellular calcium levels by optimizing the transfer of calcium ions between these two organelles. This balanced regulation of mitochondrial dynamics and calcium homeostasis was associated with improved survival and functionality of cardiomyocytes following IR injury. These findings suggest that FL3 exerts robust cardioprotective effects through its ability to promote mitochondrial fusion, enhance mitochondrial-ER interactions, and maintain calcium homeostasis. As a result, FL3 holds promise as a potential therapeutic agent for reducing myocardial damage and dysfunction associated with IR injury, offering valuable insights into novel approaches for cardioprotection.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approvals: Animal experiments were approved by Shanghai General Hospital’s Ethics Committee. All procedures complied with ARRIVE reporting standards (see Supplementary File for checklist). No human subjects, tissues, or clinical data were involved in this study; therefore, informed consent and consent for publication of human images are not applicable.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

