The dual mechanism of m6A demethylase ALKBH5 in regulating energy metabolism during exposure to MC-LR
- PMID: 40610445
- PMCID: PMC12229691
- DOI: 10.1038/s41419-025-07791-x
The dual mechanism of m6A demethylase ALKBH5 in regulating energy metabolism during exposure to MC-LR
Abstract
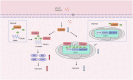
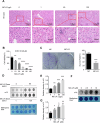
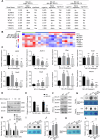
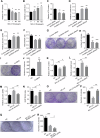
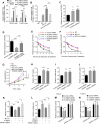
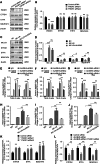
Exposure to MC-LR has been shown to cause multiple organ injury, particularly liver injury, and altered energy metabolism is closely linked. As an effective and efficient way to regulate biological gene expression, N(6)-methyladenosine(m6A) modification plays an important role in liver injury caused by microcystin-LR(MC-LR) exposure. For the first time, we reveal the dual mechanism by which AlkB homolog 5(ALKBH5) regulates energy metabolism through an m6A-YTHDF3-dependent mechanism. After MC-LR exposure, low levels of ALKBH5 increased the m6A modification of Phosphoinositide-3-Kinase Regulatory Subunit 1(PIK3R1) and m6A methylation was located at A1557. PIK3R1-m6A was recognised by YTH N6-Methyladenosine RNA Binding Protein F3(YTHDF3), which reduced the stability of PIK3R1 RNA, thereby inhibiting PIK3R1 expression and ultimately promoting glycolysis. In concert, low-level ALKBH5 inhibit oxidative phosphorylation by down-regulating the expression of Electron Transfer Flavoprotein Dehydrogenase(ETFDH), Electron Transfer Flavoprotein Subunit Alpha(ETFA) and NADH:Ubiquinone Oxidoreductase Complex Assembly Factor 4(NDUFAF4) through an m6A-YTHDF3-dependent mechanism. This dual mechanism has been shown to adversely affect cell survival in MC-LR exposed environments by significantly reducing ATP levels. This study reveals for the first time the signalling pathway and molecular mechanism of MC-LR exposure to liver injury through ALKBH5-mediated m6A modification, providing new protective and therapeutic principles.Subject terms: m6A modification; Oxidative phosphorylation; Glycolysis The mechanism of m6A demethylase ALKBH5 in regulating energy metabolism during exposure to MC-LR. Created with BioRender.com.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
ALKBH5 Regulates Osteogenic Differentiation via the lncRNA/mRNA Complex.J Dent Res. 2024 Oct;103(11):1119-1129. doi: 10.1177/00220345241266775. Epub 2024 Sep 23. J Dent Res. 2024. PMID: 39311450
-
Inhibition of ALKBH5 demethylase of m6A pathway potentiates HIV-1 reactivation from latency.Virol J. 2025 Apr 28;22(1):124. doi: 10.1186/s12985-025-02744-4. Virol J. 2025. PMID: 40296171 Free PMC article.
-
m6A demethylase ALKBH5 reduces ferroptosis in diabetic retinopathy through the m6A-YTHDF1-ACSL4 axis.Diabet Med. 2025 Aug;42(8):e70033. doi: 10.1111/dme.70033. Epub 2025 Apr 10. Diabet Med. 2025. PMID: 40210448
-
The dual-edged sword: AlkB homolog 5-mediated autophagy regulation in cancers - molecular mechanisms and therapeutic implications: A review.Int J Biol Macromol. 2025 Sep;321(Pt 1):146227. doi: 10.1016/j.ijbiomac.2025.146227. Epub 2025 Jul 21. Int J Biol Macromol. 2025. PMID: 40701469 Review.
-
Research progress of microcystin-LR toxicity to the intestine, liver, and kidney and its mechanism.Environ Int. 2025 Jul;201:109547. doi: 10.1016/j.envint.2025.109547. Epub 2025 May 29. Environ Int. 2025. PMID: 40460709 Review.
References
-
- Yang F, Huang F, Feng H, Wei J, Massey IY, Liang G, et al. A complete route for biodegradation of potentially carcinogenic cyanotoxin microcystin-LR in a novel indigenous bacterium. Water Res. 2020;174:115638. 10.1016/j.watres.2020.115638. - PubMed
-
- Buratti FM, Manganelli M, Vichi S, Stefanelli M, Scardala S, Testai E, et al. Cyanotoxins: producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch Toxicol. 2017;91:1049–130. 10.1007/s00204-016-1913-6. - PubMed
-
- Merel S, Walker D, Chicana R, Snyder S, Baures E, Thomas O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ Int. 2013;59:303–27. 10.1016/j.envint.2013.06.013. - PubMed
-
- Mugani R, El KF, Redouane EM, Haida M, Aba RP, Essadki Y, et al. Unlocking the potential of bacterioplankton-mediated microcystin degradation and removal: A bibliometric analysis of sustainable water treatment strategies. Water Res. 2024;255:121497. 10.1016/j.watres.2024.121497. - PubMed
-
- Li X, Li L, Huang Y, Wu H, Sheng S, Jiang X, et al. Upstream nitrogen availability determines the Microcystis salt tolerance and influences microcystins release in brackish water. Water Res. 2024;252:121213. 10.1016/j.watres.2024.121213. - PubMed
MeSH terms
Substances
Grants and funding
- 82404207/National Natural Science Foundation of China (National Science Foundation of China)
- 42477468/National Natural Science Foundation of China (National Science Foundation of China)
- 2024JJ6393/Natural Science Foundation of Hunan Province (Hunan Provincial Natural Science Foundation)
- 2025JJ30035/Natural Science Foundation of Hunan Province (Hunan Provincial Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

