Dynamic single-cell systemic immune responses in immunotherapy-treated early-stage HR+ breast cancer patients
- PMID: 40610460
- PMCID: PMC12229575
- DOI: 10.1038/s41523-025-00776-1
Dynamic single-cell systemic immune responses in immunotherapy-treated early-stage HR+ breast cancer patients
Abstract
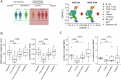
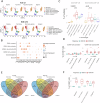
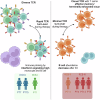
The limited added benefit of immune checkpoint inhibitors in breast cancer indicates the pressing need to identify biomarkers of response to minimize risk and maximize benefit. We used single cell RNA sequencing and T cell receptor (TCR) sequencing of peripheral blood mononuclear cells (for 28 samples comprising 79,284 cells) to monitor the peripheral immune dynamic of an exploratory cohort of hormone receptor positive breast cancer patients treated with neoadjuvant nab-paclitaxel+pembrolizumab with the ultimate goal of identifying potential peripheral blood predictive biomarkers. In responsive patients, Granzyme B positive (GZMB+) cytotoxic CD8 T cells expanded post-nab-paclitaxel+pembrolizumab, accompanied by rapid changes in TCR clones. In contrast, non-responders' peripheral T cells may experience terminal exhaustion and are not significantly altered by treatment. In addition, B cell and monocyte-specific interferon response signatures were also associated with response. Our data suggests that peripheral immunological signatures may represent a facile way to monitor dynamic antitumor immune response.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: J.M.B. receives research support from Genentech/Roche, Bristol Myers Squibb, and Incyte Corporation, has received consulting/expert witness fees from Novartis, and is an inventor on provisional patents regarding immunotherapy targets and biomarkers in cancer. E.V.A. receives research support from Novartis, Bristol Myers Squibb, Sanofi, and NextPoint, serves as advisor and consultant for Enara Bio, Manifold Bio, Monte Rosa, Novartis Institute for Biomedical Research, Serinus Bio, TracerDx, and is an inventor on patents filed on chromatin mutations and immunotherapy response, and methods for clinical interpretation and intermittent legal consulting on patents for Foaley & Hoag. A.G.W. receives research funding from Genentech, Gilead, Macrogenics, Merck and is a steering committee member for AMBRX, and serves as a consultant and paired speaker for AstraZeneca. All other authors declare no potential conflicts of interest.
Figures

References
-
- Cardoso, F. et al. LBA21 KEYNOTE-756: Phase III study of neoadjuvant pembrolizumab (pembro) or placebo (pbo) + chemotherapy (chemo), followed by adjuvant pembro or pbo + endocrine therapy (ET) for early-stage high-risk ER+/HER2– breast cancer. Ann. Oncol.34, S1260–S1261 (2023).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

