Gardenia-derived extracellular vesicles exert therapeutic effects on dopaminergic neuron apoptosis-mediated Parkinson's disease
- PMID: 40610471
- PMCID: PMC12229640
- DOI: 10.1038/s41531-025-01044-6
Gardenia-derived extracellular vesicles exert therapeutic effects on dopaminergic neuron apoptosis-mediated Parkinson's disease
Abstract
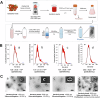
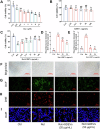
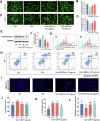
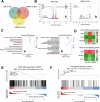
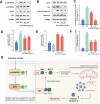
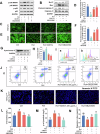
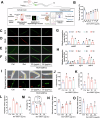
Plant-derived extracellular vesicles (EVs) show health benefits. Gardenia jasminoides Ellis, known for its neuroprotective properties, lacks therapeutic investigation on gardenia-derived extracellular vesicles (GDEVs). This study investigated the value of GDEVs in Parkinson's disease (PD) using rotenone (Rot)-induced Parkinsonism models in dopaminergic PC12 neuron cells and Caenorhabditis elegans. PD features apoptosis in dopaminergic neurons, while GDEVs alleviate PD by mitigating mitochondrial-mediated apoptosis. Specifically, GDEVs improve Rot-induced mitochondrial dysfunction to reduce cytochrome C release and apoptosis. Consequently, GDEVs reduce the risk of PD by lowering α-synuclein levels and regulating dopamine release. RNA sequencing and subsequent studies showed that GDEVs reduce p38 MAPK and p53 phosphorylation levels, and increase the Bcl-2/Bax ratio to prevent apoptosis in PC12 cells. In Caenorhabditis elegans, we verified that GDEVs reduce PD progression by increasing dopaminergic neurons using BZ555 mutants, and enhance dopamine release and motility. This study highlights the therapeutic potential of GDEVs in preventing neurodegenerative diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Lo, K. J., Wang, M. H., Ho, C. T. & Pan, M. H. Plant-derived extracellular vesicles: a new revolutionization of modern healthy diets and biomedical applications. J. Agric. Food Chem.72, 2853 (2024). - PubMed
-
- Feng, J. et al. Plant-derived vesicle-like nanoparticles as promising biotherapeutic tools: present and future. Adv. Mater.35, e2207826 (2023). - PubMed
-
- He, P. et al. Geniposide ameliorates atherosclerosis by regulating macrophage polarization via perivascular adipocyte-derived CXCL14. J. Ethnopharmacol.314, 116532 (2023). - PubMed
-
- Jin, C. et al. Gardenia (Gardenia jasminoides Ellis) fruit: a critical review of its functional nutrients, processing methods, health-promoting effects, comprehensive application and future tendencies. Crit. Rev. Food Sci. Nutr.65, 165 (2025). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

