SNAP-tag2 for faster and brighter protein labeling
- PMID: 40610720
- PMCID: PMC12568630
- DOI: 10.1038/s41589-025-01942-z
SNAP-tag2 for faster and brighter protein labeling
Abstract
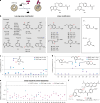
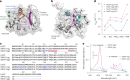
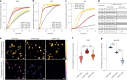
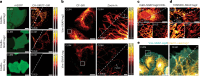
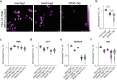
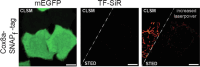
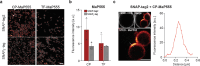
SNAP-tag is a powerful tool for labeling proteins with synthetic fluorophores in bioimaging. However, its utility in live-cell applications can be constrained by its relatively slow labeling kinetics and the limited cell permeability of its substrates. Here, we introduce improved labeling substrates and an engineered SNAP-tag for faster labeling in vitro and in live cells. SNAP-tag2 presents a second-order rate constant with rhodamine substrates that approaches 107 s-1 M-1, a 100-fold improvement over the corresponding SNAP-tag-substrate pairs. When labeled with highly fluorogenic dyes, SNAP-tag2 also shows a fivefold increase in fluorescence brightness relative to currently used SNAP-tag. The increased labeling kinetics and brightness of SNAP-tag2 translate into greatly improved performance in various live-cell (super-resolution) imaging applications.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: V.N., S.K., J.H. and K.J. are listed as inventors on a patent application (EP 24172286) on improved SNAP-tag substrates filed by the Max Planck Society. The remaining authors declare no competing interests.
Figures




References
-
- Hinner, M. J. & Johnsson, K. How to obtain labeled proteins and what to do with them. Curr. Opin. Biotechnol.21, 766–776 (2010). - PubMed
-
- Xue, L. et al. Imaging and manipulating proteins in live cells through covalent labeling. Nat. Chem. Biol.11, 917–923 (2015). - PubMed
-
- Keppler, A. et al. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat. Biotechnol.21, 86–89 (2003). - PubMed
-
- Mollwitz, B. et al. Directed evolution of the suicide protein O6-alkylguanine-DNA alkyltransferase for increased reactivity results in an alkylated protein with exceptional stability. Biochemistry51, 986–994 (2012). - PubMed
-
- Los, G. V. et al. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol.3, 373–382 (2008). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

