Prognostic model integrating histology, systemic inflammation, and recurrence status predicts immunotherapy response in advanced non-small-cell lung cancer patients
- PMID: 40611329
- PMCID: PMC12224361
- DOI: 10.1186/s13062-025-00674-3
Prognostic model integrating histology, systemic inflammation, and recurrence status predicts immunotherapy response in advanced non-small-cell lung cancer patients
Abstract
Background: Non-small-cell lung cancer (NSCLC) exhibits variable outcomes and remains a leading cause of cancer-related mortality, despite advances in immunotherapy. This study aimed to develop a prognostic model using real-world data (RWD) to stratify patients by survival outcomes and evaluate the benefit of immunotherapy across risk groups.
Methods: A retrospective cohort of 270 patients with NSCLC (2015-2024) treated with chemotherapy alone (54%) or chemoimmunotherapy (46%) was analyzed. Clinical, laboratory (neutrophil-to-lymphocyte ratio [NLR], platelet-to-lymphocyte ratio [PLR], monocyte-to-lymphocyte ratio [MLR]), and histopathological data were collected. Multivariate Cox regression identified prognostic factors for overall survival (OS) and validated them via bootstrapping.
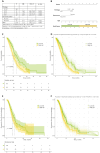
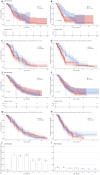
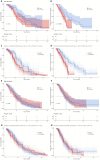
Results: The cohort (median age, 65; 78% male) had a median OS of 11.2 months and a median progression-free survival (PFS) of 7.7 months. The final prognostic model incorporated histology (adenocarcinoma vs. large cell/squamous cell carcinoma/rare subtypes: HR = 1.6-2.03), recurrence state (HR = 0.51), and NLR (HR = 1.13). Patients were stratified into low- (median OS = 14.6 months) and high-risk (median OS = 9.6 months; p < 0.001) groups. Immunotherapy significantly increased PFS in low-risk patients (12.2 vs. 7.1 months, p = 0.002) and showed an increasing trend in OS (16.9 vs. 11.3 months, p = 0.12). High-risk patients derived no OS/PFS benefit (p ≥ 0.56).
Conclusion: This RWD-derived prognostic model effectively stratifies NSCLC patients into distinct risk groups. Immunotherapy-chemotherapy provided meaningful PFS improvement in low-risk patients but minimal benefit in high-risk subgroups, underscoring the need for tailored therapeutic strategies.
Keywords: Immunotherapy; NLR; Non-small-cell lung cancer; Prognostic model.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This retrospective study included 270 patients with NSCLC from a database of N.P. Napalkov St. Petersburg Clinical Research and Practical Center of Specialized Types of Medical Care (Oncological). The study protocol was approved by the Ethics Committee of this Center (Approval No. 4 from 14.03.2023). Informed consent was waived due to the retrospective nature of the study design. Competing interests: The authors declare no competing interests. Clinical trial number: Not applicable.
Figures


References
-
- Stanley KE. Prognostic factors for survival in patients with inoperable lung Cancer. J Natl Cancer Inst. 1980;65:25–32. - PubMed
-
- Hoang T, Xu R, Schiller JH, Bonomi P, Johnson DH. Clinical model to predict survival in chemonaive patients with advanced Non-Small-Cell lung Cancer treated with Third-Generation chemotherapy regimens based on Eastern cooperative oncology group data. J Clin Oncol. 2005;23:175–83. - PubMed
-
- Heng DYC, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth Factor-Targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–9. - PubMed
-
- Lukka H, Warde P, Pickles T, Morton G, Brundage M, Souhami L. Canadian GU radiation oncologist group controversies in prostate Cancer radiotherapy: consensus development. Can J Urol. 2001;8:1314–22. - PubMed
-
- Rodríguez-Abreu D, Powell SF, Hochmair MJ, Gadgeel S, Esteban E, Felip E, Speranza G, De Angelis F, Dómine M, Cheng SY, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: Protocol-Specified final analysis from KEYNOTE-189. Ann Oncol. 2021;32:881–95. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

