Long-Term Durability of Transcatheter Aortic Valves in Patients With Bicuspid Aortic Stenosis
- PMID: 40611498
- PMCID: PMC12412356
- DOI: 10.1002/ccd.31742
Long-Term Durability of Transcatheter Aortic Valves in Patients With Bicuspid Aortic Stenosis
Abstract
Background: Data concerning the long-term durability of transcatheter aortic valves (TAVs) in patients with bicuspid aortic stenosis (AS) are lacking.
Aims: The study aims to report data on long-term valve durability following transcatheter aortic valve replacement (TAVR) in bicuspid AS.
Methods: This multicentre registry included patients who underwent TAVR for bicuspid AS with at least 2-year echocardiographic follow-up. The incidence of structural valve deterioration (SVD), bioprosthetic valve dysfunction (BVD), and bioprosthetic valve failure (BVF) was determined according to Valve Academic Research Consortium (VARC)-3 criteria.
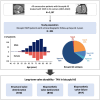
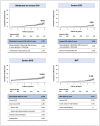
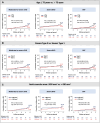
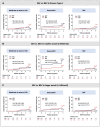
Results: Among 894 patients (mean age: 75.6 years; 39% female), the median echocardiographic follow-up was 48.7 months with a 5-year cumulative incidence of moderate-to-severe SVD, severe SVD, severe BVD, and BVF of 8.1%, 3.2%, 11.4%, and 6.1%, respectively. Younger age (≤ 75 years) was associated with a higher likelihood of reintervention (HR 2.40, log-rank p = 0.04). TAV downsizing was associated with higher rates of moderate-to-severe SVD (HR 3.05, log-rank p < 0.001), severe BVD (HR 2.07, log-rank p = 0.003), and BVF (HR 3.25, log-rank p = 0.002). In the sub-group with small annuli (area ≤ 430 mm2), implantation of balloon-expandable TAVs was associated with a higher rate of BVD in comparison with self-expanding TAVs (HR: 3.27, log-rank p = 0.008).
Conclusions: TAVs demonstrated favorable 5-year durability in patients with bicuspid AS, although younger patients were more likely to require valve reintervention. Nominal TAV sizing was associated with better durability outcomes as compared to TAV downsizing. Self-expanding valves were associated with superior hemodynamics in patients with small annuli.
Keywords: aortic stenosis; bicuspid aortic valve; transcatheter aortic valve replacement; valve durability.
© 2025 The Author(s). Catheterization and Cardiovascular Interventions published by Wiley Periodicals LLC.
Conflict of interest statement
Annette Maznyczka reports travel grants from Edwards Lifesciences, Abbott, Boston Scientific, and Medtronic. Arif Khokhar has received speaker fees from Boston Scientific and consulting fees from Machnet Medical. Stefan Toggweiler has received honoraria from Medtronic, Boston Scientific, Biosensors, Edwards Lifesciences, Hi‐D Imaging, Abbott Vascular, Medira, Shockwave, Teleflex, atHeart Medical, Cardiac Dimensions, Polares Medical, Amarin, Sanofi, AstraZeneca, ReCor Medical, Daiichi Sankyo, Bayer, Armira; has received institutional research grants from Edwards Lifesciences, Abbott Vascular, Boston Scientific, Fumedica, Novartis, Boehringer Ingelheim, Polares Medical, and holds equity in Hi‐D Imaging. Marianna Adamo has reported speaker honoraria from Abbott Vascular and Edwards Lifesciences. Matteo Montorfano received consultant fees from Abbott, Boston Scientific, Kardia, and Medtronic. Martin Swaans has received consultancy fees from Abbott Vascular, Bioventrix, Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, GE Healthcare, Medtronic, Philips Healthcare, P&F, and Siemens Healthcare. Joanna Wykrzykowska has received an institutional grant from Medtronic and speaker's honoraria (also to the institution) from Boston Scientific and Sinomed. Tau Hartikainen has received travel grants from Boston Scientific and Novartis. Stephane Noble has received an institutional grant from Abbott, consulting and speaker fees from Medtronic, and travel support to attend meetings from Edwards Lifesciences and Abbott. Darren Mylotte reports institutional research grants from Boston Scientific and Medtronic; and is a consultant for Boston Scientific, Medtronic, and MicroPort. Stephen Brecker has received grant support, consultant, and speaker fees from Medtronic. Pierfrancesco Agostoni has received consulting fees from Abbott, Boston Scientific, iVascular, Teleflex, and Terumo. Matjaž Bunc has served as a consultant or proctor for Medtronic, Edwards Lifesciences, Abbott, Medtronic, and Meril. Hélène Eltchaninoff received honoraria for lectures from Edwards Lifesciences. Daniel Blackman reports consulting fees from Medtronic Plc. and Abbott Vascular, honoraria from Medtronic Plc., Abbott Vascular, and Edwards Lifesciences, and participation in data safety/monitoring/advisory board for Medtronic Plc. and Abbott Vascular. Nicolas Van Mieghem has received institutional research grants from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, Meril, Pie Medical, PulseCath BV, Teleflex, and consultancy fees from Abbott, Abiomed, Alleviant Medical Inc., AncorValve, Anteris, Approxima Srl, Bolt Medical, Boston Scientific, Daiichi Sankyo, LUMA Vision, Materialise, Medtronic, Pie Medical, Polares, PulseCath BV, Siemens. Won‐Keun Kim reports proctor/speaker fees/advisory board participation for Abbott, Boston Scientific, Edwards Lifesciences, and Meril Life Sciences. Thomas Pilgrim reports research, travel, or educational grants to the institution without personal remuneration from Biotronik, Boston Scientific, Edwards Lifesciences, and ATSens; speaker fees and consultancy fees to the institution from Biotronik, Boston Scientific, Edwards Lifesciences, Abbott, Medtronic, Biosensors, and Highlife. Bernard Prendergast has received lecture fees from Edwards Lifesciences; and has served on a trial steering committee for Medtronic and a data safety and monitoring committee for Valvosoft. Didier Tchétché is a consultant for Abbott Vascular, Edwards Lifesciences, Medtronic, Boston Scientific, T‐Heart, and Caranx Medical. Ole De Backer received institutional research grants and consulting fees from Abbott, Boston Scientific, and Medtronic. The other authors declare no conflicts of interest.
Figures



References
-
- Roberts W. C. and Ko J. M., “Frequency by Decades of Unicuspid, Bicuspid, and Tricuspid Aortic Valves in Adults Having Isolated Aortic Valve Replacement for Aortic Stenosis, With or Without Associated Aortic Regurgitation,” Circulation 111, no. 7 (2005): 920–925, 10.1161/01.CIR.0000155623.48408.C5. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

