This is a preprint.
Identification of a conserved RNA structure in the TNFRSF1A 3'UTR: Implications for post-transcriptional regulation
- PMID: 40611903
- PMCID: PMC12224534
- DOI: 10.1101/2025.06.18.660452
Identification of a conserved RNA structure in the TNFRSF1A 3'UTR: Implications for post-transcriptional regulation
Abstract
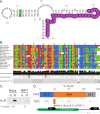
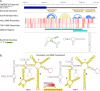
Tumor necrosis factor receptor superfamily 1A gene (TNFRSF1A) encodes the TNFR1 protein, a critical regulator of inflammation implicated in various diseases. Using ScanFold with the Integrative Genomics Viewer (IGV) GUI, we identified novel RNA structural elements within the TNFRSF1A gene. Focusing on the 3'UTR, these structures were characterized using reporter assays and targeted DMS-MaPseq. We identified a structured region that may play a role in regulating TNFR1 translation and that was also found to associate with HuR, a key regulatory RNA-binding protein. These findings provide a framework for identifying and characterizing potential functional RNA structures in therapeutically relevant genes, suggesting a new layer of post-transcriptional regulation for TNFR1 expression.
Keywords: DMS-MaPseq; HuR; RNA; TNFR1; TNFRSF1A; inflammation; structure probing; translational efficiency; untranslated region (UTR).
Figures


Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Monitoring strategies for clinical intervention studies.Cochrane Database Syst Rev. 2021 Dec 8;12(12):MR000051. doi: 10.1002/14651858.MR000051.pub2. Cochrane Database Syst Rev. 2021. PMID: 34878168 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
References
-
- Baker SJ, Reddy EP. Transducers of life and death: TNF receptor superfamily and associated proteins. Oncogene. 1996;12(1):1–9. Epub 1996/01/04. - PubMed
-
- Caminero A, Comabella M, Montalban X. Role of tumour necrosis factor (TNF)-α and TNFRSF1 R92Q mutation in the pathogenesis of TNF receptor-associated periodic syndrome and multiple sclerosis. Clinical & Experimental Immunology. 2011;166(3):338–45. doi: 10.1111/j.1365-2249.2011.04484.x. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
