When is the best time to test paratuberculosis positivity? Observations from a follow-up study in Hungarian dairy herds
- PMID: 40612149
- PMCID: PMC12225305
- DOI: 10.3389/fvets.2025.1570915
When is the best time to test paratuberculosis positivity? Observations from a follow-up study in Hungarian dairy herds
Abstract
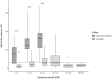
The objective of the present study was to find the most practical combination of diagnostic procedures and time points during lactation to identify Mycobacterium avium ssp. paratuberculosis (MAP)-infected animals. Four Hungarian dairy farms with a 4-5% apparent MAP positivity were enrolled in the study, and 13 non-lactating, known MAP-positive pregnant cows were chosen from each farm. Feces, blood, and milk samples were collected from each cow at 1-5, 10-14, 40-60, 90-120, 180-200, and 280-300 days in milk (DIM) and ELISA and PCR assays were performed for antibody or pathogen detection. Animals that later developed clinical paratuberculosis symptoms showed distinctly different patterns of test positivity than those that did not develop clinical symptoms during the observation period. The optimal time for detecting MAP-positive animals with the highest probability was DIM 40-60 with serum ELISA and DIM 10-14 and 40-60 for PCR assays, respectively. Serum ELISA proved to be slightly more sensitive than milk ELISA. S/P values showed a moderate correlation with the fecal qPCR Ct values. We found that the most suitable period for MAP screening is 40-60 days after calving.
Keywords: ELISA; dairy cow; paratuberculosis; qPCR; sampling time.
Copyright © 2025 Vass-Bognár, Bakony, Fornyos, Baumgartner, Khol and Jurkovich.
Conflict of interest statement
KF was employed by Eurofins Vet-Controll Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Baumgartner W, Khol JL. Paratuberculosis – potentials and limits of surveillance programs. Magy Allatorv Lapja. (2008) 130:7–10.
-
- Bakker D. Paratuberculosis control measures in Europe In: Behr MA, Collins DM, editors. Paratuberculosis: Organism, disease, control. Cambridge, MA: CAB International; (2010). 306–18.
LinkOut - more resources
Full Text Sources

