Non-Covalent Interactions and Helical Packing in Thiophene-Phenylene Copolymers: Tuning Solid-State Ordering and Charge Transport for Organic Field-Effect Transistors
- PMID: 40612493
- PMCID: PMC12225024
- DOI: 10.1021/acs.chemmater.5c00631
Non-Covalent Interactions and Helical Packing in Thiophene-Phenylene Copolymers: Tuning Solid-State Ordering and Charge Transport for Organic Field-Effect Transistors
Abstract
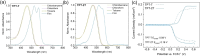
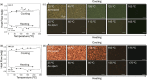
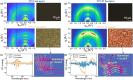
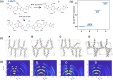
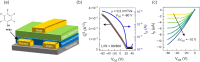
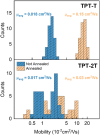
In this study, we introduce two thiophene-phenylene-thiophene (TPT) polymers designed to leverage noncovalent intramolecular interactions to regulate main-chain conformation and enhance solid-state ordering. By incorporating unsubstituted thiophene (T) or bithiophene (2T) units, we reveal striking divergence in the thermal, morphological, and optoelectronic properties of the resulting films, facilitated by these noncovalent interactions. Using a combination of computational and experimental approaches, we show that annealing yields remarkably different polymer conformations and, consequently, charge transport properties. TPT-T undergoes a significant structural transformation, adopting a more planar backbone conformation and a highly crystalline, edge-on molecular orientation. In contrast, the introduction of a single additional thiophene unit in TPT-2T leads to a more isotropic molecular orientation with a slight preference for face-on alignment, resulting in a heterogeneous film structure that hinders charge transport despite achieving tighter molecular packing. Remarkably, despite being composed of achiral components, TPT-2T develops chirality upon annealing, indicating the formation of a helical conformation. Organic field-effect transistor measurements reveal that the well-ordered alignment in annealed TPT-T films results in higher charge carrier mobility and a narrower distribution of mobility values than in TPT-2T. These findings provide critical insights into the structure-property relationships of conjugated polymers, offering guidance for optimizing molecular design and processing strategies for high-performance organic electronic materials.
© 2025 The Authors. Published by American Chemical Society.
Figures

Similar articles
-
Impact of Irreversible Adsorption on Molecular Ordering and Charge Transport in Poly(3-hexylthiophene) Thin Films on Solid Substrates.ACS Appl Mater Interfaces. 2024 Oct 16;16(41):56325-56335. doi: 10.1021/acsami.4c11049. Epub 2024 Oct 5. ACS Appl Mater Interfaces. 2024. PMID: 39368107
-
Nearly 50-50 Face-on to Edge-on Crystallites in Regioisomeric Polymers Based on n-Type Thienylvinyl-1,1-dicyanomethylene-3-indanone for High Electron Mobility.ACS Appl Mater Interfaces. 2025 Jul 9;17(27):39366-39374. doi: 10.1021/acsami.5c05769. Epub 2025 Jun 26. ACS Appl Mater Interfaces. 2025. PMID: 40574321
-
Influence of Selenium on the Optoelectronic Properties of a Series of Structurally Simple p-type Polymers for Organic Thin-Film Transistors.Macromol Rapid Commun. 2025 Jul;46(14):e2500059. doi: 10.1002/marc.202500059. Epub 2025 Apr 30. Macromol Rapid Commun. 2025. PMID: 40307167
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Psychological interventions for adults who have sexually offended or are at risk of offending.Cochrane Database Syst Rev. 2012 Dec 12;12(12):CD007507. doi: 10.1002/14651858.CD007507.pub2. Cochrane Database Syst Rev. 2012. PMID: 23235646 Free PMC article.
References
-
- Pecunia V., Petti L., Andrews J. B., Ollearo R., Gelinck G. H., Nasrollahi B., Jailani J. M., Li N., Kim J. H., Ng T. N., Feng H., Chen Z., Guo Y., Shen L., Lhuillier E., Kuo L., Sangwan V. K., Hersam M. C., Fraboni B., Basiricò L., Ciavatti A., Wu H., Niu G., Tang J., Yang G., Kim D., Dremann D., Jurchescu O. D., Bederak D., Shulga A. G., Costa P., Perinka N., Lanceros-Mendez S., Chortos A., Khuje S., Yu J., Ren S., Mascia A., Concas M., Cosseddu P., Young R. J., Yokota T., Somoya T., Jeon S. J., Zhao N., Li Y., Shukla D., Wu S., Zhu Y., Takei K., Huang Y., Spiece J., Gehring P., Persaud K., Llobet E., Krik S., Vasquez S., Costa Angeli M. A., Lugli P., Fabbri B., Spagnoli E., Rossi A., Occhipinti L. G., Tang C., Yi W., Ravenscroft D., Kandukuri T. R., Abideen Z. U., Azimi Z., Tricoli A., Rivadeneyra A., Rojas S., Gaiardo A., Valt M., Galstyan V., Zappa D., Comini E., Noël V., Mattana G., Piro B., Strand E., Bihar E., Whiting G. L., Shkodra B., Petrelli M., Moro G., Raucci A., Miglione A., Cinti S., Casson A. J., Wang Z., Bird D., Batchelor J. C., Xing L., Johnson L. S. J., Alwattar A. A., Kyndiah A., Viola F. A., Caironi M., Albarghouthi F. M., Smith B. N., Franklin A. D., Pal A., Banerjee K., Johnson Z. T., Claussen J. C., Moudgil A., Leong W. L.. Roadmap on Printable Electronic Materials for Next-Generation Sensors. Nano Futures. 2024;8(3):032001. doi: 10.1088/2399-1984/ad36ff. - DOI
-
- Zeidell A. M., Filston D. S., Waldrip M., Iqbal H. F., Chen H., McCulloch I., Jurchescu O. D.. Large-Area Uniform Polymer Transistor Arrays on Flexible Substrates: Towards High-Throughput Sensor Fabrication. Adv. Mater. Technol. 2020;5(8):2000390. doi: 10.1002/admt.202000390. - DOI
LinkOut - more resources
Full Text Sources
