Genome-wide analysis of the COMT gene family in Avena sativa: insights into lignin biosynthesis and disease defense mechanisms
- PMID: 40612596
- PMCID: PMC12222199
- DOI: 10.3389/fpls.2025.1609698
Genome-wide analysis of the COMT gene family in Avena sativa: insights into lignin biosynthesis and disease defense mechanisms
Abstract
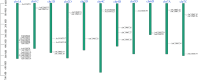
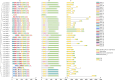
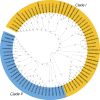
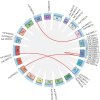
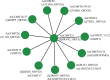
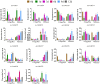
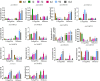
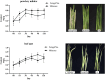
Caffeic acid O-methyltransferase (COMT) is a multifunctional enzyme involved in lignin biosynthesis and plays an important role in various primary and secondary metabolic pathways, including the plant stress response. In this study, we identified 37 AsCOMT genes from the oat (Avena sativa) whole-genome database, which are distributed across 11 chromosomes. Phylogenetic analysis grouped these genes into two major subfamilies, indicating that they are highly conserved during evolution and share close relationships with COMT genes from Zea mays and Oryza sativa. Cis-acting elements analysis revealed a rich presence of regulatory motifs related to plant hormone signaling and stress responses. Expression profiling of different oat varieties infected with powdery mildew and leaf spot disease showed significant upregulation or downregulation of several AsCOMT genes (e.g., AsCOMT14, AsCOMT22, AsCOMT24, AsCOMT27). Moreover, disease-resistant oat varieties have higher lignin contents compared to susceptible varieties. Overexpression of AsCOMT23 and AsCOMT27 in tobacco leaves resulted in significantly increased lignin content, highlighting the potential of these genes in lignin biosynthesis. These results offer a preliminary exploration of the role of AsCOMT in both lignin synthesis and the plant stress response, laying the groundwork for further functional studies and potential applications in oat breeding.
Keywords: bioinformatics analysis; caffeic acid o-methyltransferase; expression pattern; lignin biosynthesis; plant disease defense.
Copyright © 2025 Pan, Niu, Shen, Zhao, Zhang, Chai and Ju.
Conflict of interest statement
Author YZ was employed by the company Inner Mongolia Pratacultural Technology Innovation Center Co. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

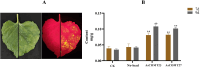
References
LinkOut - more resources
Full Text Sources
Miscellaneous

