Acute Exposure to Cadmium Triggers NCOA4-Mediated Ferritinophagy and Ferroptosis in Never-Smokers Oral Cancer Cells
- PMID: 40612671
- PMCID: PMC12223766
- DOI: 10.7150/ijbs.111228
Acute Exposure to Cadmium Triggers NCOA4-Mediated Ferritinophagy and Ferroptosis in Never-Smokers Oral Cancer Cells
Abstract
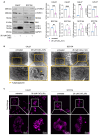
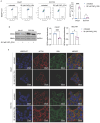
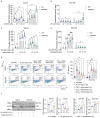
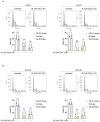
Cadmium (Cd), a carcinogenic component of tobacco, is a recognized risk factor for oral squamous cell carcinoma (OSCC). However, the molecular mechanisms underlying Cd-induced cytotoxicity in OSCC remain largely undefined. Here, we demonstrate that acute Cd exposure triggers ferroptosis in CAL27 OSCC cells derived from never-smokers, but not in SCC154 cells derived from smokers. Mechanistically, Cd outcompetes Fe, causing early iron depletion and activating the nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy. This process enhances the labile iron pool, promotes mitochondrial reactive oxygen species (ROS) generation, lipid peroxidation, and ferroptotic cell death. Notably, iron supplementation rescues CAL27 cells from Cd-induced damage, while exacerbating iron deficiency through transferrin receptor CD71 silencing amplifies cytotoxicity. Conversely, OSCC cells from smokers exhibit resistance to Cd toxicity, likely due to the overexpression of metallothionein 2A (MT2A), a heavy metal detoxification protein. Collectively, this study provides the evidence that ferritinophagy may act as a critical upstream driver of Cd-induced ferroptosis in OSCC cells derived from never-smokers, paving the way for potential ferroptosis-targeted therapeutic strategies in Cd-associated malignancies.
Keywords: Cadmium; Ferritinophagy; Ferroptosis; Iron Metabolism; NCOA4; Oral Cancer; Smokers.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- You Y, Guo Z, Wolter T, Hu Q. Intracellular metal ion-based chemistry for programmed cell death. Chem Soc Rev. 2025 Feb 3;54(3):1552–1582. - PubMed
-
- Lai Y, Gao F fen, Ge R ting, Liu R, Ma S, Liu X. Metal ions overloading and cell death. Cell Biol Toxicol. 2024;40(1):1–17. Available from: https://link.springer.com/article/10.1007/s10565-024-09910-4. - PMC - PubMed
-
- Gu J, Guo C, Ruan J, Li K, Zhou Y, Gong X. et al. From ferroptosis to cuproptosis, and calcicoptosis, to find more novel metals-mediated distinct form of regulated cell death. Apoptosis. 2024;29(5-6):586–604. Available from: https://pubmed.ncbi.nlm.nih.gov/38324163/ - PubMed
-
- Li Y, Du Y, Zhou Y, Chen Q, Luo Z, Ren Y. et al. Iron and copper: critical executioners of ferroptosis, cuproptosis and other forms of cell death. Cell Communication and Signaling 2023 21:1. 2023;21(1):1–19. Available from: https://biosignaling.biomedcentral.com/articles/10.1186/s12964-023-01267-1. - PMC - PubMed
-
- Dagdag O, Quadri TW, Haldhar R, Kim SC, Daoudi W, Berdimurodov E. et al. An Overview of Heavy Metal Pollution and Control. ACS Symposium Series. 2023;1456:3–24. Available from: https://pubs.acs.org/doi/full/10.1021/bk-2023-1456.ch001.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

