ACE-2 blockade and TMPRSS2 inhibition mitigate SARS-CoV-2 severity following cigarette smoke exposure in airway epithelial cells in vitro
- PMID: 40612711
- PMCID: PMC12221736
- DOI: 10.1016/j.omtn.2025.102580
ACE-2 blockade and TMPRSS2 inhibition mitigate SARS-CoV-2 severity following cigarette smoke exposure in airway epithelial cells in vitro
Abstract
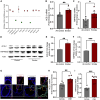
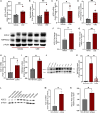
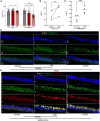
Cigarette smoking is associated with COVID-19 prevalence and severity, but the mechanistic basis for how smoking alters SARS-CoV-2 pathogenesis is unknown. A potential explanation is that smoking alters the expression of the SARS-CoV-2 cellular receptor and point of entry, angiotensin-converting enzyme 2 (ACE-2), and its cofactors including transmembrane protease serine 2 (TMPRSS2). We investigated the impact of cigarette smoking on the expression of ACE-2, TMPRSS2, and other known cofactors of SARS-CoV-2 infection and the resultant effects on infection severity in vitro. Cigarette smoke extract (CSE) exposure increased ACE-2 and TMPRSS2 mRNA expression compared to air control in ferret airway cells, Calu-3 cells, and primary human bronchial epithelial (HBE) cells derived from normal and chronic obstructive pulmonary disease (COPD) donors. CSE-exposed ferret airway cells inoculated with SARS-CoV-2 had a significantly higher intracellular viral load versus vehicle-exposed cells. Likewise, CSE exposure increased both SARS-CoV-2 intracellular viral load and viral replication in both normal and COPD HBE cells over vehicle control. Apoptosis was increased in CSE-exposed, SARS-CoV-2-infected HBE cells. Knockdown of ACE-2 via an antisense oligonucleotide (ASO) reduced SARS-CoV-2 viral load and infection in CSE-exposed ferret airway cells that was augmented by co-administration of camostat mesylate to block TMPRSS2 activity. Smoking increases SARS-CoV-2 infection via upregulation of ACE2 and TMPRSS2.
Keywords: ACE-2; ASO; CSE; MT: Oligonucleotides: Therapies and Applications; SARS-Cov2; TMPRSS2; airway epithelial cells.
© 2025 The Authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Update of
-
ACE-2 Blockade & TMPRSS2 Inhibition Mitigate SARS-CoV-2 Severity Following Cigarette Smoke Exposure in Airway Epithelial Cells In Vitro.bioRxiv [Preprint]. 2024 Jun 24:2024.06.23.600238. doi: 10.1101/2024.06.23.600238. bioRxiv. 2024. Update in: Mol Ther Nucleic Acids. 2025 Jun 02;36(3):102580. doi: 10.1016/j.omtn.2025.102580. PMID: 38979208 Free PMC article. Updated. Preprint.
Similar articles
-
ACE-2 Blockade & TMPRSS2 Inhibition Mitigate SARS-CoV-2 Severity Following Cigarette Smoke Exposure in Airway Epithelial Cells In Vitro.bioRxiv [Preprint]. 2024 Jun 24:2024.06.23.600238. doi: 10.1101/2024.06.23.600238. bioRxiv. 2024. Update in: Mol Ther Nucleic Acids. 2025 Jun 02;36(3):102580. doi: 10.1016/j.omtn.2025.102580. PMID: 38979208 Free PMC article. Updated. Preprint.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Enhancement of cigarette smoke extract-induced goblet cell metaplasia and hyperplasia exerted through IL-13 receptor α1 expression.Toxicol Lett. 2025 Jul;410:177-187. doi: 10.1016/j.toxlet.2025.06.010. Epub 2025 Jun 16. Toxicol Lett. 2025. PMID: 40532864
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
-
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
-
- Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J., Bolling M.C., Dijkstra G., Voors A.A., Osterhaus A.D., et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J. Pathol. 2020;251:228–248. doi: 10.1002/path.5471. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

